Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Gas Adsorption on Cu3(BTC)2 Metal–Organic Framework by Post-Synthetic Cation Exchange and Computational Analysis
Langmuir ( IF 3.7 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.langmuir.3c00455
José M Veleta 1 , Roy A Arrieta 1 , Yanyu Wu 1 , Miguel A Baeza 1 , Karen Castañeda 1 , Dino Villagrán 1
Langmuir ( IF 3.7 ) Pub Date : 2023-06-02 , DOI: 10.1021/acs.langmuir.3c00455
José M Veleta 1 , Roy A Arrieta 1 , Yanyu Wu 1 , Miguel A Baeza 1 , Karen Castañeda 1 , Dino Villagrán 1
Affiliation
|
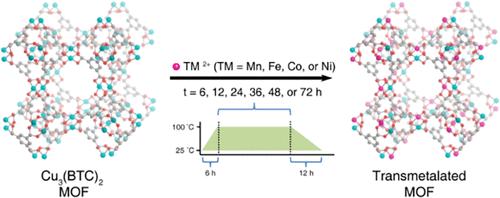
Increased gas adsorption in a series of post-synthetically modified metal–organic frameworks (MOFs) of the type HKUST-1 was achieved by the partial cation exchange process. Manipulation of post-synthetic conditions demonstrates high tunability in the site substitution and gas adsorption properties during the dynamic equilibrium process. In this work, post-synthetic modification of Cu3(BTC)2 is carried on by exposure to TM2+ solutions (TM = Mn, Fe, Co, Ni) at different time intervals. The crystal structure, composition, and morphology were studied by powder X-ray diffraction, Fourier-transform infrared spectroscopy, inductively coupled plasma optical emission spectroscopy, and scanning electron microscopy. Structural analysis supports the retention of the crystal structure and partial substitution of the Cu metal nodes within the framework. A linear increase in the transmetalation process is observed with Fe and Co with a maximum percentage of 39 and 18%, respectively. Conversely, relatively low cation exchange is observed with Mn having a maximum percentage of 2.40% and Ni with only 2.02%. Gas adsorption measurements and surface area analysis were determined for each species. Interestingly, (Cu/Mn)3(BTC)2 revealed the highest CO2 adsorption capacity of 5.47 mmol/g, compared to 3.08 mmol/g for Cu3(BTC)2. The overall increased gas adsorption can be attributed to the formation of defects in the crystal structure during the cation exchange process. These results demonstrate the outstanding potential of post-synthetic ion exchange as a general approach to fine-tuning the physical properties of existing MOF architectures.
中文翻译:

通过合成后阳离子交换和计算分析增强 Cu3(BTC)2 金属-有机骨架上的气体吸附
通过部分阳离子交换过程实现了一系列 HKUST-1 型后合成改性金属有机框架 (MOF) 中增加的气体吸附。合成后条件的操纵表明,在动态平衡过程中,位点替换和气体吸附特性具有高度可调性。在这项工作中,通过暴露于 TM 2+对 Cu 3 (BTC) 2进行合成后修饰不同时间间隔的溶液(TM = Mn、Fe、Co、Ni)。通过粉末 X 射线衍射、傅里叶变换红外光谱、电感耦合等离子体发射光谱和扫描电子显微镜研究了晶体结构、组成和形态。结构分析支持晶体结构的保留和框架内 Cu 金属节点的部分替代。观察到 Fe 和 Co 的金属转移过程呈线性增加,最大百分比分别为 39% 和 18%。相反,观察到相对较低的阳离子交换,Mn 的最大百分比为 2.40%,而 Ni 的百分比仅为 2.02%。确定每个物种的气体吸附测量和表面积分析。有趣的是,(Cu/Mn) 3 (BTC) 2与 Cu 3 (BTC) 2的 3.08 mmol/g 相比, CO 2的最高吸附容量为 5.47 mmol/g 。整体增加的气体吸附可归因于阳离子交换过程中晶体结构缺陷的形成。这些结果证明了合成后离子交换作为微调现有 MOF 结构物理特性的一般方法的突出潜力。
更新日期:2023-06-02
中文翻译:

通过合成后阳离子交换和计算分析增强 Cu3(BTC)2 金属-有机骨架上的气体吸附
通过部分阳离子交换过程实现了一系列 HKUST-1 型后合成改性金属有机框架 (MOF) 中增加的气体吸附。合成后条件的操纵表明,在动态平衡过程中,位点替换和气体吸附特性具有高度可调性。在这项工作中,通过暴露于 TM 2+对 Cu 3 (BTC) 2进行合成后修饰不同时间间隔的溶液(TM = Mn、Fe、Co、Ni)。通过粉末 X 射线衍射、傅里叶变换红外光谱、电感耦合等离子体发射光谱和扫描电子显微镜研究了晶体结构、组成和形态。结构分析支持晶体结构的保留和框架内 Cu 金属节点的部分替代。观察到 Fe 和 Co 的金属转移过程呈线性增加,最大百分比分别为 39% 和 18%。相反,观察到相对较低的阳离子交换,Mn 的最大百分比为 2.40%,而 Ni 的百分比仅为 2.02%。确定每个物种的气体吸附测量和表面积分析。有趣的是,(Cu/Mn) 3 (BTC) 2与 Cu 3 (BTC) 2的 3.08 mmol/g 相比, CO 2的最高吸附容量为 5.47 mmol/g 。整体增加的气体吸附可归因于阳离子交换过程中晶体结构缺陷的形成。这些结果证明了合成后离子交换作为微调现有 MOF 结构物理特性的一般方法的突出潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号