Synthesis ( IF 2.2 ) Pub Date : 2023-06-01 , DOI: 10.1055/s-0042-1751458 Poonsakdi Ploypradith 1, 2, 3 , Jira Jongcharoenkamol 1, 2, 4 , Kitsana Jancharoen 2 , Paratchata Batsomboon 2 , Somsak Ruchirawat 1, 2, 3
|
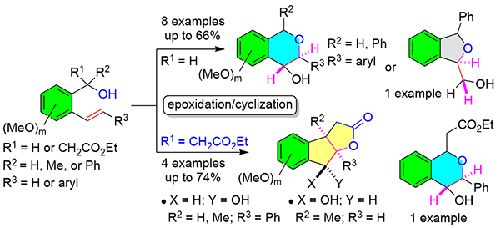
Starting from (E)-(2-stilbenyl/styrenyl)methanols, two distinct scaffolds, namely isochroman-4-ols and 1,3-dihydroisobenzofurans (phthalans), could be synthesized via an epoxidation/cyclization strategy. Indenes, readily accessible from the same starting materials, could undergo epoxidation/ring-opening/cyclization to provide tetrahydro-2H-indeno[2,1-b]furan-2-ones. Stilbene/styrene/indene epoxidation by m-CPBA or DMDO converted the nucleophilic olefin into the electrophilic epoxide, which subsequently underwent the regioselective ring-opening either by the hydroxy or the ester group to furnish the corresponding products with stereocontrol at the newly formed stereogenic centers. The reaction proceeded under substrate control to yield each product type exclusively.
中文翻译:

Isochroman-4-ols、1,3-Dihydroisobenzofurans 和 Tetrahydro-2H-indeno[2,1-b]furan-2-ones 通过 (E)-(2-芪基/苯乙烯基) 的环氧化/环化策略的发散合成甲醇
从 ( E )-(2-茋基/苯乙烯基) 甲醇开始,可以通过环氧化/环化策略合成两种不同的支架,即 isochroman-4-ols 和 1,3-dihydroisobenzofurans (phthalans)。可以从相同的起始材料轻松获得茚,可以进行环氧化/开环/环化以提供四氢-2H-茚并[2,1- b ]呋喃-2-酮。二苯乙烯/苯乙烯/茚环氧化m-CPBA 或 DMDO 将亲核烯烃转化为亲电环氧化物,随后通过羟基或酯基进行区域选择性开环,从而在新形成的立体异构中心提供具有立体控制的相应产物。反应在底物控制下进行,以排他性地产生每种产物类型。














































 京公网安备 11010802027423号
京公网安备 11010802027423号