Surface Science ( IF 2.1 ) Pub Date : 2023-06-01 , DOI: 10.1016/j.susc.2023.122336 Dalga Merve Ozkan , Ali Uzun , Burcu Selen Caglayan , Ahmet Erhan Aksoylu
|
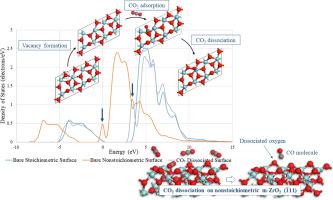
The performance stability of a Catalytic Dry Reforming of Methane (CDRM) catalyst is primarily determined by its ability to resist coke formation. This is possible through removal of carbon deposited on the hydrogen production sites of the catalyst by mobile surface oxygen formed via CO2 dissociation. In this study, it is aimed to understand the role of oxygen vacancies in activation and dissociation of CO2 molecule on m-ZrO2, which has been widely studied as the support of CDRM catalysts. For this purpose, first, oxygen vacancy formation on m-ZrO2 () surface was investigated. Two possible types of oxygen vacancies were determined based on the results of periodic DFT calculations. Then, CO2 adsorption onto both stoichiometric and nonstoichiometric m-ZrO2 () surfaces were performed. The results revealed that the CO2 dissociation is possible only on the nonstoichiometric m-ZrO2 surfaces, and that presence of an oxygen vacancy, regardless of its type, significantly lowers the adsorption energy and increases adsorbate–metal oxide interaction. The study presented here theoretically proves at atomic scale that oxygen vacancies have a great influence on the dissociative CO2 adsorption ability of m-ZrO2, and confirms its clear potential as a support to be used in technical CDRM catalysts prepared for practical applications.
中文翻译:

m-ZrO2(1¯11)上氧空位对CO2吸附和离解作用的DFT研究
甲烷催化干重整 (CDRM) 催化剂的性能稳定性主要取决于其抗焦炭形成的能力。这可以通过通过 CO 2解离形成的移动表面氧去除沉积在催化剂的氢生产位点上的碳来实现。在本研究中,旨在了解氧空位在m-ZrO 2上CO 2分子的活化和解离中的作用,m-ZrO 2 作为CDRM催化剂的载体已被广泛研究。为此,首先,在 m-ZrO 2 () 表面进行了调查。根据定期 DFT 计算的结果确定了两种可能的氧空位类型。然后,CO 2吸附到化学计量和非化学计量的 m-ZrO 2 () 表面进行。结果表明,CO 2离解仅可能发生在非化学计量的 m-ZrO 2表面,并且氧空位的存在,无论其类型如何,都会显着降低吸附能并增加吸附物与金属氧化物的相互作用。此处介绍的研究在原子尺度上从理论上证明了氧空位对m-ZrO 2的解离 CO 2吸附能力有很大影响,并证实了其作为载体的明显潜力,可用于为实际应用制备的技术 CDRM 催化剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号