当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantio- and Diastereoselective Synthesis of Chiral Tetrasubstituted α-Amino Allenoates Bearing a Vicinal All-Carbon Quaternary Stereocenter with Dual-Copper-Catalysis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-06-01 , DOI: 10.1002/anie.202305680 Cheng Sheng 1 , Zheng Ling 1 , Junzhe Xiao 2 , Kai Yang 1 , Fang Xie 1 , Shengming Ma 2, 3 , Wanbin Zhang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-06-01 , DOI: 10.1002/anie.202305680 Cheng Sheng 1 , Zheng Ling 1 , Junzhe Xiao 2 , Kai Yang 1 , Fang Xie 1 , Shengming Ma 2, 3 , Wanbin Zhang 1
Affiliation
|
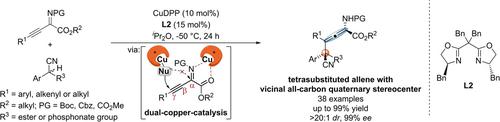
The asymmetric γ-additions of 1-alkynyl ketimines under dual-copper-catalysis have been developed, affording chiral tetrasubstituted α-amino allenoates with a vicinal all-carbon quaternary stereocenter in high yields (up to 99 % yield) with excellent stereoselectivities (up to 99 % ee, up to >20 : 1 dr). Mechanistic experiments and DFT calculations demonstrated that the asymmetric γ-addition reactions were catalyzed by double chiral Cu catalysts.
中文翻译:

双铜催化对映和非对映选择性合成带有邻位全碳四元立构中心的手性四取代α-氨基联烯酸酯
开发了双铜催化下 1-炔基酮亚胺的不对称 γ-加成反应,以高产率(高达 99% 的产率)提供具有邻位全碳季立体中心的手性四取代 α-氨基联烯酸酯,并具有优异的立体选择性(高达至 99% ee,高达 >20: 1 dr)。机理实验和DFT计算表明,双手性Cu催化剂能够催化不对称γ加成反应。
更新日期:2023-06-01
中文翻译:

双铜催化对映和非对映选择性合成带有邻位全碳四元立构中心的手性四取代α-氨基联烯酸酯
开发了双铜催化下 1-炔基酮亚胺的不对称 γ-加成反应,以高产率(高达 99% 的产率)提供具有邻位全碳季立体中心的手性四取代 α-氨基联烯酸酯,并具有优异的立体选择性(高达至 99% ee,高达 >20: 1 dr)。机理实验和DFT计算表明,双手性Cu催化剂能够催化不对称γ加成反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号