当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Umbrella Sampling Simulations of Cardiac Thin Filament Reveal Thermodynamic Consequences of Troponin I Inhibitory Peptide Mutations
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.jcim.3c00388 Austin M Cool 1 , Steffen Lindert 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-06-01 , DOI: 10.1021/acs.jcim.3c00388 Austin M Cool 1 , Steffen Lindert 1
Affiliation
|
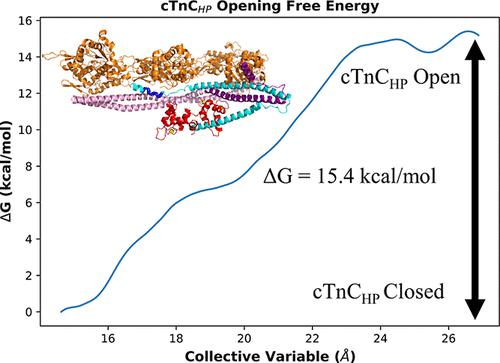
The cardiac thin filament comprises F-actin, tropomyosin, and troponin (cTn). cTn is composed of three subunits: troponin C (cTnC), troponin I (cTnI), and troponin T (cTnT). To computationally study the effect of the thin filament on cTn activation events, we employed targeted molecular dynamics followed by umbrella sampling using a model of the thin filament to measure the thermodynamics of cTn transition events. Our simulations revealed that the thin filament causes an increase in the free energy required to open the cTnC hydrophobic patch and causes a more favorable interaction between this region and the cTnI switch peptide. Mutations to the cTn complex can lead to cardiomyopathy, a collection of diseases that present clinically with symptoms of hypertrophy or dilation of the cardiac muscle, leading to impairment of the heart’s ability to function normally and ultimately myocardial infarction or heart failure. Upon introduction of cardiomyopathic mutations to R145 of cTnI, we observed a general decrease in the free energy of opening the cTnC hydrophobic patch, which is on par with previous experimental results. These mutations also exhibited a decrease in electrostatic interactions between cTnI-R145 and actin-E334. After introduction of a small molecule to the wild-type cTnI–actin interface to intentionally disrupt intersubunit contacts, we successfully observed similar thermodynamic consequences and disruptions to the same protein–protein contacts as observed with the cardiomyopathic mutations. Computational studies utilizing the cTn complex in isolation would have been unable to observe these effects, highlighting the importance of using a more physiologically relevant thin-filament model to investigate the global consequences of cardiomyopathic mutations to the cTn complex.
中文翻译:

心脏细丝的伞式采样模拟揭示肌钙蛋白 I 抑制肽突变的热力学后果
心脏细丝包含 F-肌动蛋白、原肌球蛋白和肌钙蛋白 (cTn)。 cTn 由三个亚基组成:肌钙蛋白 C (cTnC)、肌钙蛋白 I (cTnI) 和肌钙蛋白 T (cTnT)。为了通过计算研究细丝对 cTn 激活事件的影响,我们采用了目标分子动力学,然后使用细丝模型进行伞形采样来测量 cTn 转变事件的热力学。我们的模拟表明,细丝会导致打开 cTnC 疏水斑块所需的自由能增加,并导致该区域和 cTnI 开关肽之间产生更有利的相互作用。 cTn 复合物突变可导致心肌病,这是临床上表现为心肌肥大或扩张症状的一系列疾病,导致心脏正常功能受损,最终导致心肌梗塞或心力衰竭。在将心肌病突变引入 cTnI R145 后,我们观察到打开 cTnC 疏水斑块的自由能普遍下降,这与之前的实验结果一致。这些突变还表现出 cTnI-R145 和肌动蛋白-E334 之间静电相互作用的减少。在将小分子引入野生型 cTnI-肌动蛋白界面以有意破坏亚基间接触后,我们成功地观察到了与心肌病突变中观察到的类似热力学后果和对相同蛋白质-蛋白质接触的破坏。 单独利用 cTn 复合物的计算研究无法观察到这些影响,这凸显了使用生理学更相关的细丝模型来研究心肌病突变对 cTn 复合物的整体影响的重要性。
更新日期:2023-06-01
中文翻译:

心脏细丝的伞式采样模拟揭示肌钙蛋白 I 抑制肽突变的热力学后果
心脏细丝包含 F-肌动蛋白、原肌球蛋白和肌钙蛋白 (cTn)。 cTn 由三个亚基组成:肌钙蛋白 C (cTnC)、肌钙蛋白 I (cTnI) 和肌钙蛋白 T (cTnT)。为了通过计算研究细丝对 cTn 激活事件的影响,我们采用了目标分子动力学,然后使用细丝模型进行伞形采样来测量 cTn 转变事件的热力学。我们的模拟表明,细丝会导致打开 cTnC 疏水斑块所需的自由能增加,并导致该区域和 cTnI 开关肽之间产生更有利的相互作用。 cTn 复合物突变可导致心肌病,这是临床上表现为心肌肥大或扩张症状的一系列疾病,导致心脏正常功能受损,最终导致心肌梗塞或心力衰竭。在将心肌病突变引入 cTnI R145 后,我们观察到打开 cTnC 疏水斑块的自由能普遍下降,这与之前的实验结果一致。这些突变还表现出 cTnI-R145 和肌动蛋白-E334 之间静电相互作用的减少。在将小分子引入野生型 cTnI-肌动蛋白界面以有意破坏亚基间接触后,我们成功地观察到了与心肌病突变中观察到的类似热力学后果和对相同蛋白质-蛋白质接触的破坏。 单独利用 cTn 复合物的计算研究无法观察到这些影响,这凸显了使用生理学更相关的细丝模型来研究心肌病突变对 cTn 复合物的整体影响的重要性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号