当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
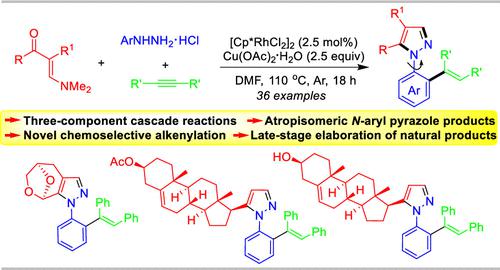
Three-Component Chemo-Selective Synthesis of N-(o-Alkenylaryl) Pyrazoles by Pyrazole Annulation and Rh-Catalyzed Chemo-Selective Aryl C–H Addition Cascade
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.joc.3c00526 Demao Chen 1 , Liyun Zhou 1 , Chengping Wen 2 , Jie-Ping Wan 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-31 , DOI: 10.1021/acs.joc.3c00526 Demao Chen 1 , Liyun Zhou 1 , Chengping Wen 2 , Jie-Ping Wan 1, 2
Affiliation
|
By using readily available enaminones, aryl hydrazine hydrochlorides, and alkynes as starting materials, the chemo-selective three-component synthesis of atropisomeric N-(o-alkenylaryl) pyrazoles has been efficiently accessed with rhodium catalysis. Unlike Satoh–Miura reaction leading to the alkyne-based C–H benzannulation by using prior prepared N-phenyl pyrazoles and alkynes as substrates, this three-component protocol displays unprecedented selectivity of C–H alkenylation by blocking the second round metal alkenylation with the key protonation step in the presence of acids.
中文翻译:

通过吡唑成环和 Rh 催化化学选择性芳基 C–H 加成级联三组分化学选择性合成 N-(邻烯基芳基) 吡唑
通过使用容易获得的烯胺酮、芳基肼盐酸盐和炔烃作为起始原料,在铑催化下有效地实现了阻转异构体N- (邻烯基芳基)吡唑的化学选择性三组分合成。与通过使用先前制备的 N-苯基吡唑和炔烃作为底物导致基于炔烃的 C-H 苯并环化的 Satoh-Miura 反应不同,该三组分方案通过用在酸存在下的关键质子化步骤。
更新日期:2023-05-31
中文翻译:

通过吡唑成环和 Rh 催化化学选择性芳基 C–H 加成级联三组分化学选择性合成 N-(邻烯基芳基) 吡唑
通过使用容易获得的烯胺酮、芳基肼盐酸盐和炔烃作为起始原料,在铑催化下有效地实现了阻转异构体N- (邻烯基芳基)吡唑的化学选择性三组分合成。与通过使用先前制备的 N-苯基吡唑和炔烃作为底物导致基于炔烃的 C-H 苯并环化的 Satoh-Miura 反应不同,该三组分方案通过用在酸存在下的关键质子化步骤。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号