Journal of Power Sources ( IF 8.1 ) Pub Date : 2023-05-31 , DOI: 10.1016/j.jpowsour.2023.233261 Yaozong Yang , Zhaolin Li , Yuesong Xu , Zhao Yang , Yang Zhang , Jie Wang , Hong Xu , Xiangming He , Hailei Zhao
|
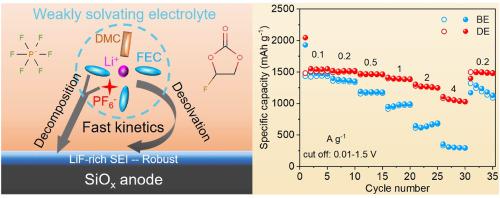
The growing demand for electric vehicles has led to an urgent need for higher-energy-density batteries. Silicon (Si) has been regarded as an alternative to graphite as an anode material owing to its high theoretical capacity. Nevertheless, the large volume change in Si during repeated charge/discharge causes serious particle fracture, leading to rapid capacity degradation. Silicon suboxide (SiOx) anodes show better cycling performance than Si because of their alleviated volume variation, yet still have unsatisfactory electrode reaction kinetics. Herein, fluoroethylene carbonate (FEC)-based electrolyte is proposed to enhance the electrochemical properties of SiOx anodes. A higher specific capacity of ∼1500 mAh g−1 at 0.5 A g−1 and an excellent rate capability of ∼1050 mAh g−1 at 4 A g−1 are obtained for the SiOx anode, which ensures an 83.5% capacity retention of SiOx||LiFePO4 cells after 300 cycles. The theoretical calculations and experimental studies reveal that FEC forms a weakly solvating electrolyte, enabling easier Li-ion desolvation and fast electrode reaction kinetics. Moreover, FEC reinforces the Li+-anion coordination owing to its weak solvating capability, thus a LiF-rich electrode/electrolyte interphase is formed through the co-decomposition of FEC and PF6− anions, leading to a tough SEI film formation with rapid ionic conductivity and good mechanical property.
中文翻译:

基于氟代碳酸亚乙酯的电解质促进低氧化硅阳极的快速电极反应动力学
对电动汽车不断增长的需求导致对更高能量密度电池的迫切需求。硅 (Si) 由于其高理论容量而被视为石墨的替代负极材料。然而,在反复充电/放电过程中 Si 的大体积变化会导致严重的颗粒破裂,从而导致容量快速下降。低氧化硅 (SiO x ) 阳极显示出比 Si 更好的循环性能,因为它们减少了体积变化,但仍然具有不令人满意的电极反应动力学。在此,提出了基于氟代碳酸亚乙酯 (FEC) 的电解质来增强 SiO x阳极的电化学性能。 在 0.5 A g -1时更高的比容量为 ∼1500 mAh g -1SiO x负极 在 4 A g -1时获得了~1050 mAh g -1的优异倍率性能,这确保了 SiO x ||LiFePO 4电池在 300 次循环后的容量保持率为 83.5%。理论计算和实验研究表明,FEC 形成弱溶剂化电解质,使锂离子去溶剂化更容易,电极反应动力学更快。此外,由于 FEC 的弱溶剂化能力,FEC 加强了 Li + - 阴离子的配位,因此通过 FEC 和 PF 6 -的共分解形成富含 LiF 的电极/电解质界面。阴离子,导致形成具有快速离子电导率和良好机械性能的坚韧SEI膜。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号