Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-05-30 , DOI: 10.1016/j.cej.2023.143772 Seongbin Jo , Han Dong Son , Tae-Young Kim , Jin Hyeok Woo , Do Yeong Ryu , Jae Chang Kim , Soo Chool Lee , Kandis Leslie Gilliard-AbdulAziz
|
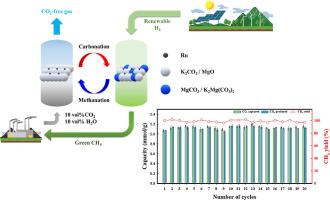
In this study, we have developed a Ru/K2CO3–MgO (Ru/KMg) catal-sorbent for integrated CO2 capture and methanation (ICCM) at low temperatures. The Ru primarily existed as a K2RuO3 phase, which was not observed after reduction at 400 °C. In addition, the crystallite size of Ru0 is smaller than that of Ru/MgO because Ru species as K2RuO3 phase, instead of RuO2, is dispersed well throughout the MgO support material. Here, the CO2 capture and regeneration properties of Ru/KMg catal-sorbents after carbonation at different temperatures (60, 120, 150, and 320 °C) were studied under N2 or H2 conditions, respectively. The optimal carbonation temperature was 150 °C when considering 100% CO2 conversion to CH4. 20 consecutive cycles of ICCM were conducted at 150 °C for carbonation (10 vol% CO2 and 10 vol% H2O) and 320 °C for methanation (90 vol% H2). The results showed stable CH4 productivities of 1.07–1.19 mmol CH4/g with 100% CH4 selectivity and 96.2%–101.3% CH4 yield.
中文翻译:

Ru/K2CO3–MgO 催化吸附剂,用于低温下集成的 CO2 捕获和甲烷化
在这项研究中,我们开发了一种 Ru/K 2 CO 3 –MgO (Ru/KMg) 催化吸附剂,用于在低温下集成 CO 2捕获和甲烷化 (ICCM)。Ru 主要以 K 2 RuO 3相存在,在 400 °C 还原后未观察到。此外,Ru 0的微晶尺寸小于Ru/MgO的微晶尺寸,因为作为K 2 RuO 3相的Ru物质,而不是RuO 2,很好地分散在整个MgO载体材料中。在此,研究了在不同温度(60、120、150 和 320 °C)下碳酸化后 Ru/KMg 催化剂吸附剂在 N2 下的CO 2捕获和再生性能2或 H 2条件,分别。当考虑将 100% CO 2转化为 CH 4时,最佳碳酸化温度为 150 °C 。在 150 °C 进行碳酸化(10 vol% CO 2和 10 vol% H 2 O)和 320 °C 进行甲烷化(90 vol% H 2)的条件下进行 20 个连续的 ICCM 循环。结果显示稳定的 CH 4生产率为 1.07–1.19 mmol CH 4 /g,CH 4选择性为 100% ,CH 4产率为 96.2%–101.3%。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号