Synthesis ( IF 2.2 ) Pub Date : 2023-05-30 , DOI: 10.1055/a-2081-1830 Joshua D. Tibbetts , Alexander J. Cresswell , Hannah E. Askey , Qiao Cao , James D. Grayson , Sophie L. Hobson , George D. Johnson , Jacob C. Turner-Dore
|
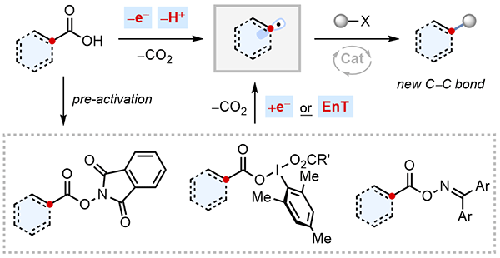
The ubiquity of carboxylic acids as naturally derived or man-made chemical feedstocks has spurred the development of powerful, decarboxylative C–C bond-forming transformations for organic synthesis. Carboxylic acids benefit not only from extensive commercial availability, but are stable surrogates for organohalides or organometallic reagents in transition-metal-catalysed cross-coupling. Open shell reactivity of carboxylic acids (or derivatives thereof) to furnish carbon-centred radicals is proving transformative for synthetic chemistry, enabling novel and strategy-level C(sp3)–C bond disconnections with exquisite chemoselectivity. This short review will summarise several of the latest advances in this ever-expanding area.
1 Introduction
2 Improved Decarboxylative Arylations
3 sp3–sp3 Cross-Coupling of Carboxylic Acids with Aliphatic Bromides
4 sp3–sp3 Cross-Coupling of Carboxylic Acids with Aliphatic Alcohols and Amines
5 Doubly Decarboxylative sp3–sp3 Cross-Coupling of Carboxylic Acids
6 Decarboxylative C–C Bond Formation from (Hetero)aryl Carboxylic Acids
7 Conclusions
中文翻译:

与烷基或芳基羧酸脱羧、自由基 C-C 键形成:最新进展
羧酸作为天然衍生或人造化学原料的普遍存在促进了有机合成中强大的脱羧 C-C 键形成转化的发展。羧酸不仅受益于广泛的商业可用性,而且在过渡金属催化的交叉偶联中是有机卤化物或有机金属试剂的稳定替代品。羧酸(或其衍生物)提供以碳为中心的自由基的开壳反应性被证明对合成化学具有变革性,使新颖和战略级的 C(sp 3 )–C 键断开具有精致的化学选择性。这篇简短的评论将总结这个不断扩展的领域的一些最新进展。
1 简介
2 改进的脱羧芳基化
3 sp 3 –sp 3羧酸与脂肪族溴化物的交叉偶联
4 sp 3 –sp 3羧酸与脂肪醇和胺的交叉偶联
5 双脱羧 sp 3 –sp 3羧酸的交叉偶联
6 (杂)芳基羧酸的脱羧 C-C 键形成
7 结论













































 京公网安备 11010802027423号
京公网安备 11010802027423号