当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing Phase-Transfer Catalysts and a Phosphoramidite Ligand via the Asymmetric Intramolecular Hydroamination Reaction
Organometallics ( IF 2.5 ) Pub Date : 2023-05-30 , DOI: 10.1021/acs.organomet.3c00124
Daven Foster 1 , Stephen A. Moggach 1 , Reto Dorta 1
Organometallics ( IF 2.5 ) Pub Date : 2023-05-30 , DOI: 10.1021/acs.organomet.3c00124
Daven Foster 1 , Stephen A. Moggach 1 , Reto Dorta 1
Affiliation
|
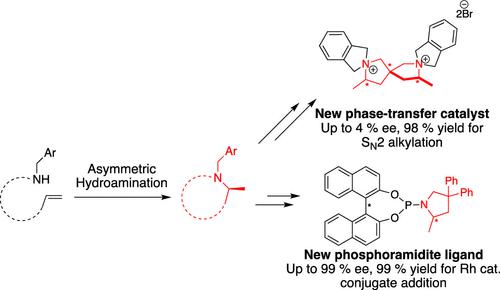
Within the last few years, our group has developed the intramolecular, asymmetric hydroamination (HA) reaction to a point where essentially enantiopure products can be obtained. The question then arose as to whether we can apply the reaction in useful ways beyond the synthesis of natural/pharmaceutically relevant products. In the present paper, we incorporate a select few HA products for the production of new chiral phase-transfer catalysts. These species are synthesized in one or two synthetic steps from new spirocyclic HA products and are applied in a classical substitution reaction, showing negligible enantioselectivity. In a second application, we introduce a novel phosphoramidite ligand, obtained by combining BINOL (1,1′-bi-2-naphthol) and an optically pure HA product with phosphorous trichloride. The representative ligand shows high enantioselectivity and activity in the rhodium-catalyzed conjugate addition reaction (Hayashi–Miyaura reaction).
中文翻译:

通过不对称分子内氢氨化反应获得相转移催化剂和亚磷酰胺配体
在过去的几年里,我们的团队开发了分子内不对称氢胺化(HA)反应,基本上可以得到对映体纯的产品。接下来的问题是,除了天然/药物相关产品的合成之外,我们是否可以以有用的方式应用该反应。在本文中,我们结合了一些精选的 HA 产品来生产新型手性相转移催化剂。这些物质是由新的螺环 HA 产品通过一两个合成步骤合成的,并应用于经典的取代反应,显示出可忽略不计的对映选择性。在第二个应用中,我们介绍了一种新型亚磷酰胺配体,该配体是通过将 BINOL(1,1'-双-2-萘酚)和光学纯 HA 产品与三氯化磷结合而获得的。
更新日期:2023-05-30
中文翻译:

通过不对称分子内氢氨化反应获得相转移催化剂和亚磷酰胺配体
在过去的几年里,我们的团队开发了分子内不对称氢胺化(HA)反应,基本上可以得到对映体纯的产品。接下来的问题是,除了天然/药物相关产品的合成之外,我们是否可以以有用的方式应用该反应。在本文中,我们结合了一些精选的 HA 产品来生产新型手性相转移催化剂。这些物质是由新的螺环 HA 产品通过一两个合成步骤合成的,并应用于经典的取代反应,显示出可忽略不计的对映选择性。在第二个应用中,我们介绍了一种新型亚磷酰胺配体,该配体是通过将 BINOL(1,1'-双-2-萘酚)和光学纯 HA 产品与三氯化磷结合而获得的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号