Journal of Power Sources ( IF 8.1 ) Pub Date : 2023-05-27 , DOI: 10.1016/j.jpowsour.2023.233249 Shouquan Zhou , Siyu Zhang , Shang Wang , Weiling Zhang , Yan Liu , Hui Lin , Jingjing Chen , Longfei Yan , Fuweng Zhang , Haohong Li , Huidong Zheng
|
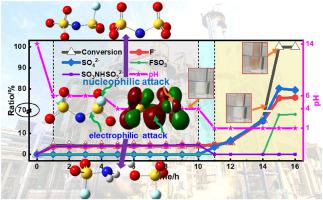
LiFSI (lithium bis(fluorosulfonyl)imide) is a promising lithium salt for electrolytes in Li-ion batteries. However, the accumulation of harmful gases and heat during LiFSI hydrolysis could lead to serious safety accidents. Here we systematically investigate LiFSI hydrolysis processes under comprehensive conditions: higher temperature/acidity/basicity and lower water content can accelerate the hydrolysis, whereas the presence of DEC (diethyl carbonate) solvent, and other alkali metals (Na+, K+) can stabilize FSI−. Unexpectedly, under alkaline conditions, temperature/water content could not affect the hydrolysis greatly. By monitoring the hydrolysis intermediates and products using time-dependent ion chromatography, infrared spectra, and nuclear magnetic resonance, the hydrolysis routes are proposed and validated by accelerating rate calorimetry, differential scanning calorimetry measurements, and theoretical calculations. Under neutral/acidic conditions, electrophilic attack on the S–N bond generates FSO2NH2 and FSO3−, while nucleophilic attack on the S–F bond produces FSO2NSO32− and SO3NHSO32− under alkaline conditions. As indicated by DFT calculation, the weaker S–N bond and larger S–N–S angle facilitate the electrophilic attack under acid conditions. Furthermore, very unstable intermediates (FSO2NH2 and CH3CH2OSO3H) are determined for the first time. Based on these hydrolysis mechanisms, strategies for inhibiting LiFSI hydrolysis are provided, which is significant for the high-efficiency production and safe storage/transportation of LiFSI.
中文翻译:

工业生产中双(氟磺酰基)亚胺阴离子水解的直接证据:基于热力学分析和理论模拟的途径
LiFSI(双(氟磺酰基)亚胺锂)是一种很有前途的锂离子电池电解质锂盐。然而,LiFSI水解过程中有害气体和热量的积累可能导致严重的安全事故。在这里,我们系统地研究了综合条件下的 LiFSI 水解过程:较高的温度/酸度/碱度和较低的水含量可以加速水解,而 DEC(碳酸二乙酯)溶剂和其他碱金属(Na +,K +)的存在可以稳定FSI -. 出乎意料的是,在碱性条件下,温度/含水量不会对水解产生很大影响。通过使用时间依赖性离子色谱、红外光谱和核磁共振监测水解中间体和产物,提出了水解路线并通过加速量热法、差示扫描量热法测量和理论计算进行了验证。在中性/酸性条件下,对 S–N 键的亲电攻击生成 FSO 2 NH 2和 FSO 3 −,而对 S–F 键的亲核攻击生成 FSO 2 NSO 3 2−和 SO 3 NHSO 3 2−在碱性条件下。如 DFT 计算所示,较弱的 S-N 键和较大的 S-N-S 角促进了酸性条件下的亲电攻击。此外,首次测定了非常不稳定的中间体(FSO 2 NH 2和 CH 3 CH 2 OSO 3 H)。基于这些水解机制,提供了抑制LiFSI水解的策略,这对于LiFSI的高效生产和安全储存/运输具有重要意义。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号