Joule ( IF 38.6 ) Pub Date : 2023-05-26 , DOI: 10.1016/j.joule.2023.05.003
Geonhui Lee , Armin Sedighian Rasouli , Byoung-Hoon Lee , Jinqiang Zhang , Da Hye Won , Yurou Celine Xiao , Jonathan P. Edwards , Mi Gyoung Lee , Eui Dae Jung , Fatemeh Arabyarmohammadi , Hengzhou Liu , Ivan Grigioni , Jehad Abed , Tartela Alkayyali , Shijie Liu , Ke Xie , Rui Kai Miao , Sungjin Park , Roham Dorakhan , Yong Zhao , Colin P. O’Brien , Zhu Chen , David Sinton , Edward Sargent
|
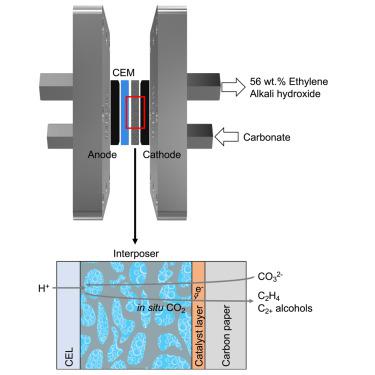
Alkali hydroxide systems capture CO2 as carbonate; however, generating a pure CO2 stream requires significant energy input, typically from thermal cycling to 900°C. What is more, the subsequent valorization of gas-phase CO2 into products presents additional energy requirements and system complexities, including managing the formation of (bi)carbonate in an electrolyte and separating unreacted CO2 downstream. Here, we report the direct electrochemical conversion of CO2, captured in the form of carbonate, into multicarbon (C2+) products. Using an interposer and a Cu/CoPc-CNTs electrocatalyst, we achieve 47% C2+ Faradaic efficiency at 300 mA cm−2 and a full cell voltage of 4.1 V. We report 56 wt % of C2H4 and no detectable C1 gas in the product gas stream: CO, CH4, and CO2 combined total below 0.9 wt % (0.1 vol %). This approach obviates the need for energy to regenerate lost CO2, an issue seen in prior CO2-to-C2+ reports.
中文翻译:

从碳酸盐捕集液中将 CO2 电还原为多碳产品
碱金属氢氧化物系统将 CO 2捕获为碳酸盐;然而,产生纯 CO 2流需要大量能量输入,通常从热循环到 900°C。更重要的是,随后将气相 CO 2稳定化为产品会带来额外的能源需求和系统复杂性,包括管理电解液中碳酸氢盐的形成以及在下游分离未反应的 CO 2。在这里,我们报告了以碳酸盐形式捕获的CO 2直接电化学转化为多碳 (C 2+ ) 产物。使用中介层和 Cu/CoPc-CNTs 电催化剂,我们在 300 mA cm -2下实现了 47% C 2+法拉第效率和 4.1 V 的全电池电压。我们报告了 56 wt% 的 C 2 H 4并且在产品气流中没有检测到 C 1气体:CO、CH 4和 CO 2 的总和低于 0.9 wt % (0.1 vol %) . 这种方法避免了再生损失的 CO 2所需的能量,这是之前 CO 2 -to-C 2+报告中出现的一个问题。

































 京公网安备 11010802027423号
京公网安备 11010802027423号