Structure ( IF 4.4 ) Pub Date : 2023-05-25 , DOI: 10.1016/j.str.2023.05.001 Arka Prabha Ray 1 , Naveen Thakur 1 , Niloofar Gopal Pour 1 , Matthew T Eddy 1
|
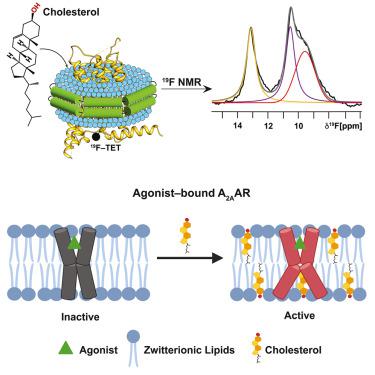
Cholesterol is a critical component of mammalian cell membranes and an allosteric modulator of G protein-coupled receptors (GPCRs), but divergent views exist on the mechanisms by which cholesterol influences receptor functions. Leveraging the benefits of lipid nanodiscs, i.e., quantitative control of lipid composition, we observe distinct impacts of cholesterol in the presence and absence of anionic phospholipids on the function-related conformational dynamics of the human A2A adenosine receptor (A2AAR). Direct receptor-cholesterol interactions drive activation of agonist-bound A2AAR in membranes containing zwitterionic phospholipids. Intriguingly, the presence of anionic lipids attenuates cholesterol’s impact through direct interactions with the receptor, highlighting a more complex role for cholesterol that depends on membrane phospholipid composition. Targeted amino acid replacements at two frequently predicted cholesterol interaction sites showed distinct impacts of cholesterol at different receptor locations, demonstrating the ability to delineate different roles of cholesterol in modulating receptor signaling and maintaining receptor structural integrity.
中文翻译:

依赖于膜磷脂组成的胆固醇-GPCR 相互作用的双重机制
胆固醇是哺乳动物细胞膜的重要组成部分,也是 G 蛋白偶联受体 (GPCR) 的变构调节剂,但对于胆固醇影响受体功能的机制存在不同看法。利用脂质纳米盘的优点,即脂质成分的定量控制,我们观察到阴离子磷脂存在和不存在时胆固醇对人 A 2A腺苷受体 (A 2A AR) 功能相关构象动力学的明显影响。直接受体-胆固醇相互作用驱动含有两性离子磷脂的膜中激动剂结合的 A 2A AR 的激活。有趣的是,阴离子脂质的存在通过与受体的直接相互作用减弱了胆固醇的影响,突显了胆固醇取决于膜磷脂组成的更复杂的作用。两个经常预测的胆固醇相互作用位点的靶向氨基酸替换显示出胆固醇对不同受体位置的不同影响,证明了描绘胆固醇在调节受体信号传导和维持受体结构完整性方面的不同作用的能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号