当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of Monobromamine and Dibromamine with Phenolic Compounds and Organic Matter: Kinetics and Formation of Bromophenols and Bromoform
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.est.3c00935 Anette T Mensah 1 , Yingying Xiang 1 , Florence Berne 1 , Sylvie Soreau 2 , Hervé Gallard 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-05-25 , DOI: 10.1021/acs.est.3c00935 Anette T Mensah 1 , Yingying Xiang 1 , Florence Berne 1 , Sylvie Soreau 2 , Hervé Gallard 1
Affiliation
|
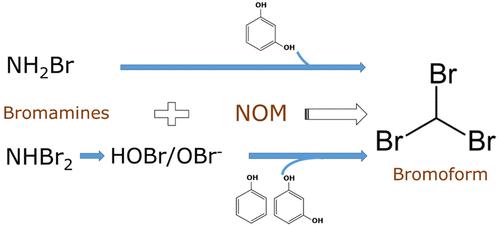
Monobromamine (NH2Br) and dibromamine (NHBr2) produced from reactions of hypobromous acid (HOBr) with ammonia can react with phenolic structures of natural organic matter (NOM) to produce disinfection byproducts such as bromoform (CHBr3). The reactivity of NH2Br was controlled by the reaction of the bromoammonium ion (NH3Br+) with phenolate species, with specific rate constants ranging from 6.32 × 102 for 2,4,6-tribromophenol to 1.22 × 108 M–1 s–1 for phenol. Reactions of NHBr2 with phenol and bromophenols were negligible compared to its self-decomposition; rate constants could be determined only with resorcinol for pH > 7. At pH 8.1–8.2, no formation of CHBr3 was observed from the reaction of NH2Br with phenol while the reaction of NH2Br with resorcinol produced a significant concentration of CHBr3. In contrast to NH2Br, a significant amount of CHBr3 produced with an excess of NHBr2 over phenol was explained by the reactions of HOBr produced from NHBr2 decomposition. A comprehensive kinetic model including the formation and decomposition of bromamines and the reactivity of HOBr and NH2Br with phenolic compounds was developed at pH 8.0–8.3. Furthermore, the kinetic model was used to evaluate the significance of the NH2Br and NHBr2 reactions with the phenolic structures of two NOM isolates.
中文翻译:

一溴胺和二溴胺与酚类化合物和有机物的反应:动力学以及溴酚和三溴甲烷的形成
次溴酸(HOBr )与氨反应产生的一溴胺( NH 2 Br)和二溴胺(NHBr 2)可与天然有机物(NOM)的酚结构反应,产生消毒副产物,如三溴甲烷(CHBr 3)。NH 2 Br的反应性由溴铵离子 (NH 3 Br + ) 与酚盐物质的反应控制,具体速率常数范围为 2,4,6-三溴苯酚的 6.32 × 10 2到 1.22 × 10 8 M –苯酚为1 s –1 。NHBr 2与苯酚和溴苯酚的反应与其自分解相比可以忽略不计;仅当 pH > 7 时,才能用间苯二酚测定速率常数。在 pH 8.1–8.2 时, NH 2 Br 与苯酚的反应没有观察到CHBr 3的形成,而 NH 2 Br 与间苯二酚的反应产生了显着浓度的 CHBr 3 . 与NH 2 Br相反,用相对于苯酚过量的NHBr 2产生显着量的CHBr 3是通过NHBr 2分解产生的HOBr的反应来解释的。在 pH 8.0–8.3 下开发了一个综合动力学模型,包括溴胺的形成和分解以及 HOBr 和 NH 2 Br 与酚类化合物的反应性。此外,该动力学模型还用于评估 NH 2 Br 和 NHBr 2反应与两种 NOM 分离物酚结构的显着性。
更新日期:2023-05-25
中文翻译:

一溴胺和二溴胺与酚类化合物和有机物的反应:动力学以及溴酚和三溴甲烷的形成
次溴酸(HOBr )与氨反应产生的一溴胺( NH 2 Br)和二溴胺(NHBr 2)可与天然有机物(NOM)的酚结构反应,产生消毒副产物,如三溴甲烷(CHBr 3)。NH 2 Br的反应性由溴铵离子 (NH 3 Br + ) 与酚盐物质的反应控制,具体速率常数范围为 2,4,6-三溴苯酚的 6.32 × 10 2到 1.22 × 10 8 M –苯酚为1 s –1 。NHBr 2与苯酚和溴苯酚的反应与其自分解相比可以忽略不计;仅当 pH > 7 时,才能用间苯二酚测定速率常数。在 pH 8.1–8.2 时, NH 2 Br 与苯酚的反应没有观察到CHBr 3的形成,而 NH 2 Br 与间苯二酚的反应产生了显着浓度的 CHBr 3 . 与NH 2 Br相反,用相对于苯酚过量的NHBr 2产生显着量的CHBr 3是通过NHBr 2分解产生的HOBr的反应来解释的。在 pH 8.0–8.3 下开发了一个综合动力学模型,包括溴胺的形成和分解以及 HOBr 和 NH 2 Br 与酚类化合物的反应性。此外,该动力学模型还用于评估 NH 2 Br 和 NHBr 2反应与两种 NOM 分离物酚结构的显着性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号