当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalyzed Dowd–Beckwith radical-polar crossover reaction for the synthesis of medium-sized carbocyclic compounds
Chemical Science ( IF 7.6 ) Pub Date : 2023-05-25 , DOI: 10.1039/d3sc01908j Tushar Singha 1 , Ganesh Arjun Kadam 1 , Durga Prasad Hari 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-05-25 , DOI: 10.1039/d3sc01908j Tushar Singha 1 , Ganesh Arjun Kadam 1 , Durga Prasad Hari 1
Affiliation
|
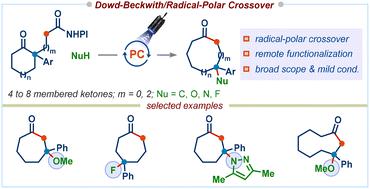
The Dowd–Beckwith reaction, a ring-expansion of carbonyl compounds via alkoxy radicals, is a powerful approach for synthesizing medium to large-sized carbocyclic scaffolds, which takes advantage of existing ring structures and avoids entropic and enthalpic factors that arise from the end-to-end cyclization strategies. However, the Dowd–Beckwith ring-expansion followed by H-atom abstraction is still the dominating pathway, which hampers its synthetic applications, and there currently exist no reports on the functionalization of ring-expanded radicals using non-carbon based nucleophilic reagents. Herein, we report a redox-neutral decarboxylative Dowd–Beckwith/radical-polar crossover (RPC) sequence that delivers functionalized medium-sized carbocyclic compounds with broad functional group tolerance. The reaction allows one-carbon ring-expansion of 4-, 5-, 6-, 7-, and 8-membered ring substrates and can also be applied to three-carbon chain incorporation, enabling remote functionalization in medium-sized rings.
中文翻译:

光催化Dowd-Beckwith自由基-极性交叉反应合成中等碳环化合物
Dowd-Beckwith 反应,羰基化合物的扩环反应烷氧基自由基是合成中型至大型碳环支架的有效方法,它利用现有的环结构并避免端到端环化策略产生的熵和焓因素。然而,Dowd-Beckwith扩环后夺取H原子仍然是主要途径,这阻碍了其合成应用,并且目前还没有使用非碳基亲核试剂对扩环自由基进行官能化的报道。在此,我们报道了一种氧化还原中性脱羧Dowd-Beckwith/自由基-极性交叉(RPC)序列,该序列可提供具有广泛官能团耐受性的功能化中等大小碳环化合物。该反应允许 4-、5-、6-、7-、
更新日期:2023-05-25
中文翻译:

光催化Dowd-Beckwith自由基-极性交叉反应合成中等碳环化合物
Dowd-Beckwith 反应,羰基化合物的扩环反应烷氧基自由基是合成中型至大型碳环支架的有效方法,它利用现有的环结构并避免端到端环化策略产生的熵和焓因素。然而,Dowd-Beckwith扩环后夺取H原子仍然是主要途径,这阻碍了其合成应用,并且目前还没有使用非碳基亲核试剂对扩环自由基进行官能化的报道。在此,我们报道了一种氧化还原中性脱羧Dowd-Beckwith/自由基-极性交叉(RPC)序列,该序列可提供具有广泛官能团耐受性的功能化中等大小碳环化合物。该反应允许 4-、5-、6-、7-、


















































 京公网安备 11010802027423号
京公网安备 11010802027423号