当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reverse Dual-Ion Battery Enabled by Reversing the Cation/Anion Storage Mechanism in an Aqueous ZnCl2 Water-in-Salt Electrolyte
ChemPhysChem ( IF 2.3 ) Pub Date : 2023-05-23 , DOI: 10.1002/cphc.202300098 Asis Sethi 1, 2 , Anil Kumar U 1, 2 , Vishal M Dhavale 1, 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2023-05-23 , DOI: 10.1002/cphc.202300098 Asis Sethi 1, 2 , Anil Kumar U 1, 2 , Vishal M Dhavale 1, 2
Affiliation
|
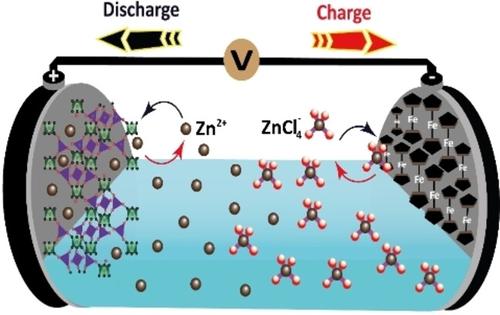
Demonstrated the remarkable capability of a reverse dual-ion battery comprising a low-redox potential anode and a cation-deficient cathode material to alter the ion de(insertion) chemistry in ZnCl2 water-in-salt electrolyte. Specifically, this innovative design has revolutionized the sequencing chemistry of ions-born, showcasing its potential for ground-breaking advancements in energy storage technology.
中文翻译:

通过反转 ZnCl2 盐包水电解质中的阳离子/阴离子存储机制实现反向双离子电池
展示了包含低氧化还原电位阳极和缺阳离子阴极材料的反向双离子电池改变 ZnCl 2盐包水电解质中离子脱(插入)化学的卓越能力。具体来说,这种创新设计彻底改变了离子生成的测序化学,展示了其在储能技术方面取得突破性进步的潜力。
更新日期:2023-05-23
中文翻译:

通过反转 ZnCl2 盐包水电解质中的阳离子/阴离子存储机制实现反向双离子电池
展示了包含低氧化还原电位阳极和缺阳离子阴极材料的反向双离子电池改变 ZnCl 2盐包水电解质中离子脱(插入)化学的卓越能力。具体来说,这种创新设计彻底改变了离子生成的测序化学,展示了其在储能技术方面取得突破性进步的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号