当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic Binding of ATP and Nucleic Acids Necessitates UPF1’s ATPase Functional Cycle
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.jcim.3c00247
Bin Sun 1 , Te Liu 1 , Manjie Zhang 1 , Shuijie Li 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-05-24 , DOI: 10.1021/acs.jcim.3c00247
Bin Sun 1 , Te Liu 1 , Manjie Zhang 1 , Shuijie Li 2
Affiliation
|
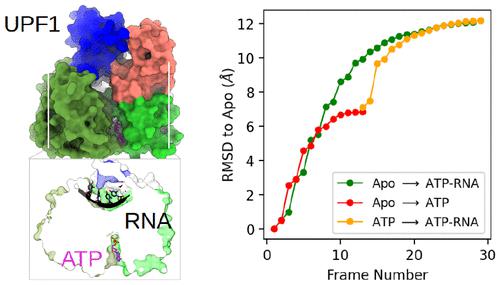
UPF1 is a core protein in the nonsense mRNA degradation (NMD) surveillance pathway that degrades aberrant mRNA. UPF1 has both ATPase and RNA helicase activities, but it exhibits mutually exclusive binding of ATP and RNA. This suggests intricate allosteric coupling between ATP and RNA binding that remains unresolved. In this study, we used molecular dynamics simulations and dynamic network analyses to probe the dynamics and free energy landscapes covering UPF1 crystal structures resolved in the Apo state, the ATP bound state, and the ATP-RNA bound (catalytic transition) state. Free energy calculations show that in the presence of ATP and RNA, the transition from the Apo state to the ATP bound state is an uphill process but becomes a downhill process when transitioning to the catalytic transition state. Allostery potential analyses reveal that the Apo and catalytic transition states are mutually allosterically activated toward each other, reflecting the intrinsic ATPase function of UPF1. The Apo state is also allosterically activated toward the ATP bound state. However, binding ATP alone leads to an allosterically trapped state that is difficult to revert to either the Apo or the catalytic transition state. The high allostery potential of Apo UPF1 toward different states results in a “first come, first served” mechanism that requires the synergistic binding of ATP and RNA to drive the ATPase cycle. Our results reconcile UPF1’s ATPase and RNA helicase activities within an allostery framework and may apply to other SF1 helicases, as we demonstrate that UPF1’s allostery signaling pathways prefer the RecA1 domain over the equally fold-conserved RecA2 domain, and this preference coincides with higher sequence conservation in the RecA1 domain across typical human SF1 helicases.
中文翻译:

ATP 和核酸的协同结合需要 UPF1 的 ATP 酶功能循环
UPF1 是无义 mRNA 降解 (NMD) 监视通路中的核心蛋白,可降解异常 mRNA。UPF1 同时具有 ATP 酶和 RNA 解旋酶活性,但它表现出 ATP 和 RNA 的相互排斥结合。这表明 ATP 和 RNA 结合之间错综复杂的变构耦合仍未解决。在这项研究中,我们使用分子动力学模拟和动态网络分析来探索涵盖在 Apo 状态、ATP 结合状态和 ATP-RNA 结合(催化转变)状态下解析的 UPF1 晶体结构的动力学和自由能景观。自由能计算表明,在 ATP 和 RNA 存在的情况下,从 Apo 态到 ATP 结合态的转变是一个上坡过程,但当转变到催化过渡态时,则变成一个下坡过程。变构电位分析表明,Apo 和催化过渡态相互变构激活,反映了 UPF1 的内在 ATPase 功能。Apo 状态也向 ATP 结合状态变构激活。然而,单独结合 ATP 会导致难以恢复到 Apo 或催化过渡态的变构捕获状态。Apo UPF1 对不同状态的高变构潜力导致“先到先得”机制,需要 ATP 和 RNA 的协同结合来驱动 ATPase 循环。我们的结果在变构框架内调和了 UPF1 的 ATP 酶和 RNA 解旋酶活性,并可能适用于其他 SF1 解旋酶,
更新日期:2023-05-24
中文翻译:

ATP 和核酸的协同结合需要 UPF1 的 ATP 酶功能循环
UPF1 是无义 mRNA 降解 (NMD) 监视通路中的核心蛋白,可降解异常 mRNA。UPF1 同时具有 ATP 酶和 RNA 解旋酶活性,但它表现出 ATP 和 RNA 的相互排斥结合。这表明 ATP 和 RNA 结合之间错综复杂的变构耦合仍未解决。在这项研究中,我们使用分子动力学模拟和动态网络分析来探索涵盖在 Apo 状态、ATP 结合状态和 ATP-RNA 结合(催化转变)状态下解析的 UPF1 晶体结构的动力学和自由能景观。自由能计算表明,在 ATP 和 RNA 存在的情况下,从 Apo 态到 ATP 结合态的转变是一个上坡过程,但当转变到催化过渡态时,则变成一个下坡过程。变构电位分析表明,Apo 和催化过渡态相互变构激活,反映了 UPF1 的内在 ATPase 功能。Apo 状态也向 ATP 结合状态变构激活。然而,单独结合 ATP 会导致难以恢复到 Apo 或催化过渡态的变构捕获状态。Apo UPF1 对不同状态的高变构潜力导致“先到先得”机制,需要 ATP 和 RNA 的协同结合来驱动 ATPase 循环。我们的结果在变构框架内调和了 UPF1 的 ATP 酶和 RNA 解旋酶活性,并可能适用于其他 SF1 解旋酶,

































 京公网安备 11010802027423号
京公网安备 11010802027423号