Life Sciences ( IF 5.2 ) Pub Date : 2023-05-23 , DOI: 10.1016/j.lfs.2023.121793 Zhengshan Gao 1 , Honghong Zhan 1 , Wei Zong 1 , Miaomiao Sun 1 , Lang Linghu 1 , Guowei Wang 1 , Fancheng Meng 1 , Min Chen 1
|
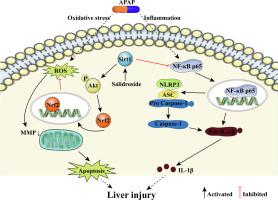
对乙酰氨基酚 (APAP) 过量引起的肝毒性是全世界药物性肝损伤的最常见原因,与氧化应激和无菌性炎症显着相关。红景天苷是从红景天中提取的主要活性成分L.,具有抗氧化和抗炎活性。在此,我们研究了红景天苷对 APAP 诱导的肝损伤的保护作用及其潜在机制。红景天苷预处理逆转了 APAP 对 L02 细胞中细胞活力、LDH 释放和细胞凋亡的影响。此外,红景天苷可以逆转APAP引起的ROS积累和MMP崩溃现象。红景天苷提高核 Nrf2、HO-1 和 NQO1 的水平。利用PI3k/Akt抑制剂LY294002进一步证实红景天苷通过Akt途径介导Nrf2核转位。用 Nrf2 siRNA 或 LY294002 预处理可显着阻止红景天苷的抗凋亡作用。此外,红景天苷还可降低 APAP 升高的核 NF-κB、NLRP3、ASC、裂解的 caspase-1 和成熟 IL-1β 的水平。而且,红景天苷预处理增加了Sirt1的表达,而Sirt1敲低则减弱了红景天苷的保护活性,同时逆转了红景天苷介导的Akt/Nrf2通路的上调和NF-κB/NLRP3炎症小体轴的下调。然后我们使用C57BL/6小鼠建立APAP诱导的肝损伤模型,发现红景天苷显着减轻肝损伤。此外,蛋白质印迹分析表明,在 APAP 处理的小鼠中,红景天苷可促进 Sirt1 表达,激活 Akt/Nrf2 通路,并抑制 NF-κB/NLRP3 炎性体轴。本研究的结果支持红景天苷在改善 APAP 诱导的肝毒性中的可能应用。同时逆转红景天苷介导的 Akt/Nrf2 通路上调和 NF-κB/NLRP3 炎性体轴下调。然后我们使用C57BL/6小鼠建立APAP诱导的肝损伤模型,发现红景天苷显着减轻肝损伤。此外,蛋白质印迹分析表明,在 APAP 处理的小鼠中,红景天苷可促进 Sirt1 表达,激活 Akt/Nrf2 通路,并抑制 NF-κB/NLRP3 炎性体轴。本研究的结果支持红景天苷在改善 APAP 诱导的肝毒性中的可能应用。同时逆转红景天苷介导的 Akt/Nrf2 通路上调和 NF-κB/NLRP3 炎性体轴下调。然后我们使用C57BL/6小鼠建立APAP诱导的肝损伤模型,发现红景天苷显着减轻肝损伤。此外,蛋白质印迹分析表明,在 APAP 处理的小鼠中,红景天苷可促进 Sirt1 表达,激活 Akt/Nrf2 通路,并抑制 NF-κB/NLRP3 炎性体轴。本研究的结果支持红景天苷在改善 APAP 诱导的肝毒性中的可能应用。然后我们使用C57BL/6小鼠建立APAP诱导的肝损伤模型,发现红景天苷显着减轻肝损伤。此外,蛋白质印迹分析表明,在 APAP 处理的小鼠中,红景天苷可促进 Sirt1 表达,激活 Akt/Nrf2 通路,并抑制 NF-κB/NLRP3 炎性体轴。本研究的结果支持红景天苷在改善 APAP 诱导的肝毒性中的可能应用。然后我们使用C57BL/6小鼠建立APAP诱导的肝损伤模型,发现红景天苷显着减轻肝损伤。此外,蛋白质印迹分析表明,在 APAP 处理的小鼠中,红景天苷可促进 Sirt1 表达,激活 Akt/Nrf2 通路,并抑制 NF-κB/NLRP3 炎性体轴。本研究的结果支持红景天苷在改善 APAP 诱导的肝毒性中的可能应用。

"点击查看英文标题和摘要"































 京公网安备 11010802027423号
京公网安备 11010802027423号