Corrosion Science ( IF 7.4 ) Pub Date : 2023-05-23 , DOI: 10.1016/j.corsci.2023.111288 X. Wang , W.L. Xu , Z.Y. Liu , G.A. Zhang
|
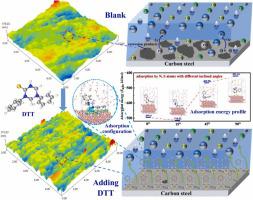
Triazine derivatives, 3-(4-thioxo-1,3,5-triazinan-1-yl) propanoic acid (TPA) and 1,5-diphenyl-1,3,5-triazinane-2-thione (DTT), were synthesized by a one-step synthetic process and utilized as high efficiency corrosion inhibitors for carbon steel (CS) in the oil production. Experimental tests demonstrate that both TPA and DTT show superior inhibition performance, with inhibition efficiencies (IEs) of 97.8% and 99.1%, respectively. GFN-xTB calculations reveal the adsorptions of TPA and DTT on CS surface through the S, N, O atoms of TPA, or the S, N atoms and phenyl ring of DTT. The most stable adsorption configurations of TPA and DTT are at an inclined angle of 15o between the thiourea fragment and steel surface, which are determined with different initial adsorption configurations. The presence of phenyl ring in DTT not only endows it with stronger hydrophobicity, but also facilitates its strong absorption on CS surface by forming multiple Fe–C bonds through the π-electrons in the phenyl ring, which accounts for the better inhibitive effect of DTT. Additionally, the presence of H2O solvent deteriorates the adsorptions of TPA and DTT due to the weak interactions between the TPA/DTT and H2O molecules, which is demonstrated by the more positive adsorption energy and longer bonding distances of TPA and DTT on Fe surface.
中文翻译:

揭示三嗪衍生物对碳钢的缓蚀机理:界面吸附的实验和理论见解
三嗪衍生物 3-(4-thioxo-1,3,5-triazinan-1-yl) propanoic acid (TPA) 和 1,5-diphenyl-1,3,5-triazinane-2-thione (DTT)通过一步合成工艺合成,并在石油生产中用作碳钢 (CS) 的高效缓蚀剂。实验测试表明,TPA 和 DTT 均表现出优异的抑制性能,抑制效率 (IE) 分别为 97.8% 和 99.1%。GFN-xTB 计算揭示了 TPA 和 DTT 通过 TPA 的 S、N、O 原子或 DTT 的 S、N 原子和苯环吸附在 CS 表面。TPA 和 DTT 最稳定的吸附构型是 15 o的倾斜角硫脲碎片和钢表面之间的关系,这是由不同的初始吸附构型决定的。DTT中苯环的存在不仅赋予其更强的疏水性,而且通过苯环中的π电子形成多个Fe-C键,促进其在CS表面的强吸附,从而使DTT具有更好的抑制效果. 此外,由于 TPA/DTT 和 H 2 O 分子之间的弱相互作用,H 2 O 溶剂的存在会降低 TPA 和 DTT 的吸附,这可以通过 TPA 和 DTT 在表面上更正的吸附能和更长的键合距离来证明。铁表面。






























 京公网安备 11010802027423号
京公网安备 11010802027423号