Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The adhesion GPCRs CELSR1–3 and LPHN3 engage G proteins via distinct activation mechanisms
Cell Reports ( IF 7.5 ) Pub Date : 2023-05-23 , DOI: 10.1016/j.celrep.2023.112552
Duy Lan Huong Bui 1 , Andrew Roach 1 , Jingxian Li 2 , Sumit J Bandekar 2 , Elizabeth Orput 1 , Ritika Raghavan 1 , Demet Araç 2 , Richard C Sando 1
Cell Reports ( IF 7.5 ) Pub Date : 2023-05-23 , DOI: 10.1016/j.celrep.2023.112552
Duy Lan Huong Bui 1 , Andrew Roach 1 , Jingxian Li 2 , Sumit J Bandekar 2 , Elizabeth Orput 1 , Ritika Raghavan 1 , Demet Araç 2 , Richard C Sando 1
Affiliation
|
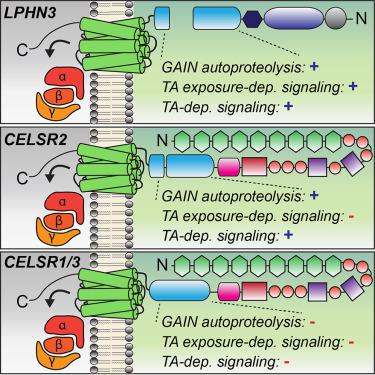
Adhesion G protein-coupled receptors (aGPCRs) are a large GPCR class that direct diverse fundamental biological processes. One prominent mechanism for aGPCR agonism involves autoproteolytic cleavage, which generates an activating, membrane-proximal tethered agonist (TA). How universal this mechanism is for all aGPCRs is unclear. Here, we investigate G protein induction principles of aGPCRs using mammalian latrophilin 3 (LPHN3) and cadherin EGF LAG-repeat 7-transmembrane receptors 1–3 (CELSR1–3), members of two aGPCR families conserved from invertebrates to vertebrates. LPHNs and CELSRs mediate fundamental aspects of brain development, yet CELSR signaling mechanisms are unknown. We find that CELSR1 and CELSR3 are cleavage deficient, while CELSR2 is efficiently cleaved. Despite differential autoproteolysis, CELSR1–3 all engage GαS, and CELSR1 or CELSR3 TA point mutants retain GαS coupling activity. CELSR2 autoproteolysis enhances GαS coupling, yet acute TA exposure alone is insufficient. These studies support that aGPCRs signal via multiple paradigms and provide insights into CELSR biological function.
中文翻译:

粘附 GPCR CELSR1-3 和 LPHN3 通过不同的激活机制与 G 蛋白结合
粘附 G 蛋白偶联受体 (aGPCR) 是一个大型 GPCR 类别,可指导多种基本生物过程。 aGPCR 激动的一个重要机制涉及自蛋白水解裂解,它会产生一种活化的近膜束缚激动剂 (TA)。该机制对于所有 aGPCR 的普遍性尚不清楚。在这里,我们使用哺乳动物 latrophilin 3 (LPHN3) 和钙粘蛋白 EGF LAG 重复 7 跨膜受体 1-3 (CELSR1-3) 研究 aGPCR 的 G 蛋白诱导原理,这两个受体是从无脊椎动物到脊椎动物保守的两个 aGPCR 家族的成员。 LPHN 和 CELSR 介导大脑发育的基本方面,但 CELSR 信号传导机制尚不清楚。我们发现 CELSR1 和 CELSR3 裂解缺陷,而 CELSR2 裂解有效。尽管自蛋白水解作用存在差异,CELSR1-3 均与 GαS 结合,并且 CELSR1 或 CELSR3 TA 点突变体保留了 GαS 偶联活性。 CELSR2 自身蛋白水解增强了 GαS 偶联,但仅急性 TA 暴露是不够的。这些研究支持 aGPCR 通过多种范例发出信号,并提供了对 CELSR 生物学功能的见解。
更新日期:2023-05-23
中文翻译:

粘附 GPCR CELSR1-3 和 LPHN3 通过不同的激活机制与 G 蛋白结合
粘附 G 蛋白偶联受体 (aGPCR) 是一个大型 GPCR 类别,可指导多种基本生物过程。 aGPCR 激动的一个重要机制涉及自蛋白水解裂解,它会产生一种活化的近膜束缚激动剂 (TA)。该机制对于所有 aGPCR 的普遍性尚不清楚。在这里,我们使用哺乳动物 latrophilin 3 (LPHN3) 和钙粘蛋白 EGF LAG 重复 7 跨膜受体 1-3 (CELSR1-3) 研究 aGPCR 的 G 蛋白诱导原理,这两个受体是从无脊椎动物到脊椎动物保守的两个 aGPCR 家族的成员。 LPHN 和 CELSR 介导大脑发育的基本方面,但 CELSR 信号传导机制尚不清楚。我们发现 CELSR1 和 CELSR3 裂解缺陷,而 CELSR2 裂解有效。尽管自蛋白水解作用存在差异,CELSR1-3 均与 GαS 结合,并且 CELSR1 或 CELSR3 TA 点突变体保留了 GαS 偶联活性。 CELSR2 自身蛋白水解增强了 GαS 偶联,但仅急性 TA 暴露是不够的。这些研究支持 aGPCR 通过多种范例发出信号,并提供了对 CELSR 生物学功能的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号