当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Morphology-Dictated Mechanism of Efficient Reaction Sites for Li2O2 Decomposition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-22 , DOI: 10.1021/jacs.2c12267 Hao Yan 1 , Wei-Wei Wang 1 , Tai-Rui Wu 1 , Yu Gu 1 , Kai-Xuan Li 1 , De-Yin Wu 1 , MingSen Zheng 1 , Quanfeng Dong 1 , Jiawei Yan 1 , Bing-Wei Mao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-22 , DOI: 10.1021/jacs.2c12267 Hao Yan 1 , Wei-Wei Wang 1 , Tai-Rui Wu 1 , Yu Gu 1 , Kai-Xuan Li 1 , De-Yin Wu 1 , MingSen Zheng 1 , Quanfeng Dong 1 , Jiawei Yan 1 , Bing-Wei Mao 1
Affiliation
|
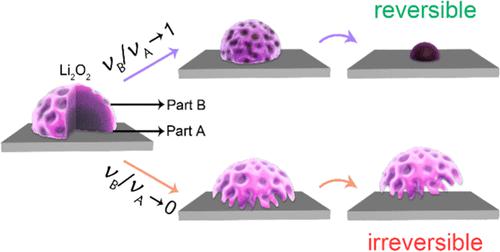
In the pursuit of a highly reversible lithium–oxygen (Li–O2) battery, control of reaction sites to maintain stable conversion between O2 and Li2O2 at the cathode side is imperatively desirable. However, the mechanism involving the reaction site during charging remains elusive, which, in turn, imposes challenges in recognition of the origin of overpotential. Herein, via combined investigations by in situ atomic force microscopy (AFM) and electrochemical impedance spectroscopy (EIS), we propose a universal morphology-dictated mechanism of efficient reaction sites for Li2O2 decomposition. It is found that Li2O2 deposits with different morphologies share similar localized conductivities, much higher than that reported for bulk Li2O2, enabling the reaction site not only at the electrode/Li2O2/electrolyte interface but also at the Li2O2/electrolyte interface. However, while the mass transport process is more enhanced at the former, the charge-transfer resistance at the latter is sensitively related to the surface structure and thus the reactivity of the Li2O2 deposit. Consequently, for compact disk-like deposits, the electrode/Li2O2/electrolyte interface serves as the dominant decomposition site, which causes premature departure of Li2O2 and loss of reversibility; on the contrary, for porous flower-like and film-like Li2O2 deposits bearing a larger surface area and richer surface-active structures, both the interfaces are efficient for decomposition without premature departure of the deposit so that the overpotential arises primarily from the sluggish oxidation kinetics and the decomposition is more reversible. The present work provides instructive insights into the understanding of the mechanism of reaction sites during the charge process, which offers guidance for the design of reversible Li–O2 batteries.
中文翻译:

Li2O2 分解有效反应位点的形态决定机制
在追求高度可逆的锂-氧 (Li-O 2 ) 电池时,控制反应位点以在阴极侧保持 O 2和 Li 2 O 2之间的稳定转化是当务之急。然而,充电过程中涉及反应位点的机制仍然难以捉摸,这反过来又对识别过电势的来源提出了挑战。在此,通过原位原子力显微镜 (AFM) 和电化学阻抗谱 (EIS) 的联合研究,我们提出了 Li 2 O 2 分解有效反应位点的通用形态学决定机制。发现 Li 2 O 2具有不同形态的沉积物具有相似的局部电导率,远高于报告的块状 Li 2 O 2的电导率,使反应位点不仅在电极/Li 2 O 2 /电解质界面而且在 Li 2 O 2 /电解质界面。然而,虽然前者的传质过程更加增强,但后者的电荷转移阻力与表面结构敏感相关,因此与 Li 2 O 2 沉积物的反应性相关。因此,对于致密盘状沉积物,电极/Li 2 O 2/电解质界面是主要的分解位点,导致Li 2 O 2过早脱离和可逆性丧失;相反,对于具有更大表面积和更丰富的表面活性结构的多孔花状和膜状 Li 2 O 2沉积物,两个界面都可以有效分解而不会过早离开沉积物,因此过电势主要来自缓慢的氧化动力学和分解更可逆。目前的工作为理解充电过程中反应位点的机制提供了有益的见解,为可逆 Li-O 2电池的设计提供了指导。
更新日期:2023-05-22
中文翻译:

Li2O2 分解有效反应位点的形态决定机制
在追求高度可逆的锂-氧 (Li-O 2 ) 电池时,控制反应位点以在阴极侧保持 O 2和 Li 2 O 2之间的稳定转化是当务之急。然而,充电过程中涉及反应位点的机制仍然难以捉摸,这反过来又对识别过电势的来源提出了挑战。在此,通过原位原子力显微镜 (AFM) 和电化学阻抗谱 (EIS) 的联合研究,我们提出了 Li 2 O 2 分解有效反应位点的通用形态学决定机制。发现 Li 2 O 2具有不同形态的沉积物具有相似的局部电导率,远高于报告的块状 Li 2 O 2的电导率,使反应位点不仅在电极/Li 2 O 2 /电解质界面而且在 Li 2 O 2 /电解质界面。然而,虽然前者的传质过程更加增强,但后者的电荷转移阻力与表面结构敏感相关,因此与 Li 2 O 2 沉积物的反应性相关。因此,对于致密盘状沉积物,电极/Li 2 O 2/电解质界面是主要的分解位点,导致Li 2 O 2过早脱离和可逆性丧失;相反,对于具有更大表面积和更丰富的表面活性结构的多孔花状和膜状 Li 2 O 2沉积物,两个界面都可以有效分解而不会过早离开沉积物,因此过电势主要来自缓慢的氧化动力学和分解更可逆。目前的工作为理解充电过程中反应位点的机制提供了有益的见解,为可逆 Li-O 2电池的设计提供了指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号