当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkali–Amide-Catalyzed One-Pot Aminoallylation of Aldehydes with Allylbenzenes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-22 , DOI: 10.1021/acs.joc.3c00581 Yuanyun Gu 1 , Yan-En Wang 2 , Yaqi Yuan 1 , Haodong Xu 1 , Yizhou Lu 1 , Yufei Zhang 1 , Fei Xue 3 , Dan Xiong , Jianyou Mao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-05-22 , DOI: 10.1021/acs.joc.3c00581 Yuanyun Gu 1 , Yan-En Wang 2 , Yaqi Yuan 1 , Haodong Xu 1 , Yizhou Lu 1 , Yufei Zhang 1 , Fei Xue 3 , Dan Xiong , Jianyou Mao 1
Affiliation
|
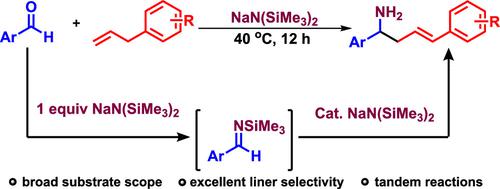
The deprotonation of allylbenzene was successfully demonstrated with a catalytic alkali amide base (NaN(SiMe3)2). The deprotonated allyl anion could be trapped by in situ generated N-(trimethylsilyl) aldimines to provide value-added homoallylic amines (39 examples, 68–98% yields) in a one-pot manner with excellent liner selectivity. Compared with the previously reported method for the synthesis of homoallylic amines, this method does not need to use the preinstalled protection groups on the imines, which need to be removed after the reaction to obtain the N–H free homoallylic amine derivatives.
中文翻译:

碱-酰胺催化的醛与烯丙基苯的一锅氨基烯丙基化反应
用催化性碱金属酰胺碱 (NaN(SiMe 3 ) 2 ) 成功证明了烯丙基苯的去质子化。去质子化的烯丙基阴离子可以被原位生成的N- (三甲基甲硅烷基) 醛亚胺捕获,以一锅法提供具有优异线性选择性的增值同烯丙基胺(39 个实例,68-98% 产率)。与之前报道的合成高烯丙基胺的方法相比,该方法不需要使用亚胺上预先设置的保护基团,反应后需要将其去除以获得N-H游离的高烯丙基胺衍生物。
更新日期:2023-05-22
中文翻译:

碱-酰胺催化的醛与烯丙基苯的一锅氨基烯丙基化反应
用催化性碱金属酰胺碱 (NaN(SiMe 3 ) 2 ) 成功证明了烯丙基苯的去质子化。去质子化的烯丙基阴离子可以被原位生成的N- (三甲基甲硅烷基) 醛亚胺捕获,以一锅法提供具有优异线性选择性的增值同烯丙基胺(39 个实例,68-98% 产率)。与之前报道的合成高烯丙基胺的方法相比,该方法不需要使用亚胺上预先设置的保护基团,反应后需要将其去除以获得N-H游离的高烯丙基胺衍生物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号