当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Large Uncertainties in the Thermodynamics of Phosphorus (III) Oxide (P4O6) Have Significant Implications for Phosphorus Species in Planetary Atmospheres
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-05-22 , DOI: 10.1021/acsearthspacechem.3c00016
William Bains 1, 2, 3 , Matthew A. Pasek 4 , Sukrit Ranjan 5 , Janusz J. Petkowski 1, 6 , Arthur Omran 4 , Sara Seager 1, 7, 8
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2023-05-22 , DOI: 10.1021/acsearthspacechem.3c00016
William Bains 1, 2, 3 , Matthew A. Pasek 4 , Sukrit Ranjan 5 , Janusz J. Petkowski 1, 6 , Arthur Omran 4 , Sara Seager 1, 7, 8
Affiliation
|
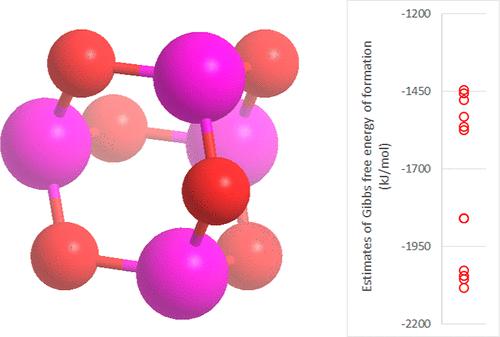
Phosphorus (III) oxide (P4O6) has been suggested to be a major component of the gas phase phosphorus chemistry in the atmospheres of gas giant planets and of Venus. However, P4O6’s proposed role is based on thermodynamic modeling, itself based on values for the free energy of formation of P4O6 estimated from limited experimental data. Values of the standard Gibbs free energy of formation (ΔGo(g)) of P4O6 in the literature differ by up to ∼656 kJ/mol, a huge range. Depending on which value is assumed, P4O6 may either be the majority phosphorus species present or be completely absent from modeled atmospheres. Here, we critically review the literature thermodynamic values and compare their predictions to observed constraints on P4O6 geochemistry. We conclude that the widely used values from the NIST/JANAF database are almost certainly too low (predicting that P4O6 is more stable than is plausible). We show that, regardless of the value of ΔGo(g) for P4O6 assumed, the formation of phosphine from P4O6 in the Venusian atmosphere is thermodynamically unfavorable. We conclude that there is a need for more robust data on both the thermodynamics of phosphorus chemistry for astronomical and geological modeling in general and for understanding the atmosphere of Venus and the gas giant planets in particular.
中文翻译:

氧化磷 (III) (P4O6) 热力学的巨大不确定性对行星大气中的磷物质具有重大影响
氧化磷(III)(P 4 O 6)被认为是气态巨行星和金星大气中气相磷化学的主要成分。然而,P 4 O 6的提议作用是基于热力学模型,其本身基于根据有限的实验数据估计的 P 4 O 6形成自由能的值。文献中P 4 O 6的标准吉布斯生成自由能(ΔG o (g) ) 的值相差高达∼656 kJ/mol,范围很大。根据假设的值,P 4 O 6可能是模拟大气中存在的主要磷物质,也可能完全不存在。在这里,我们批判性地回顾了文献热力学值,并将其预测与观察到的 P 4 O 6地球化学约束进行了比较。我们得出的结论是,NIST/JANAF 数据库中广泛使用的值几乎肯定太低(预测 P 4 O 6比看似合理的更稳定)。我们表明,无论假定P 4 O 6的 Δ Go (g)值如何,P 4 O 6都会形成磷化氢金星大气层中的空气在热力学上是不利的。我们的结论是,一般天文和地质建模以及了解金星和气态巨行星的大气层都需要更可靠的磷化学热力学数据。
更新日期:2023-05-22
中文翻译:

氧化磷 (III) (P4O6) 热力学的巨大不确定性对行星大气中的磷物质具有重大影响
氧化磷(III)(P 4 O 6)被认为是气态巨行星和金星大气中气相磷化学的主要成分。然而,P 4 O 6的提议作用是基于热力学模型,其本身基于根据有限的实验数据估计的 P 4 O 6形成自由能的值。文献中P 4 O 6的标准吉布斯生成自由能(ΔG o (g) ) 的值相差高达∼656 kJ/mol,范围很大。根据假设的值,P 4 O 6可能是模拟大气中存在的主要磷物质,也可能完全不存在。在这里,我们批判性地回顾了文献热力学值,并将其预测与观察到的 P 4 O 6地球化学约束进行了比较。我们得出的结论是,NIST/JANAF 数据库中广泛使用的值几乎肯定太低(预测 P 4 O 6比看似合理的更稳定)。我们表明,无论假定P 4 O 6的 Δ Go (g)值如何,P 4 O 6都会形成磷化氢金星大气层中的空气在热力学上是不利的。我们的结论是,一般天文和地质建模以及了解金星和气态巨行星的大气层都需要更可靠的磷化学热力学数据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号