Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-05-19 , DOI: 10.1016/j.bioorg.2023.106615 Marina Roussaki 1 , George E Magoulas 1 , Theano Fotopoulou 1 , Nuno Santarem 2 , Emile Barrias 3 , Ina Pöhner 4 , Sara Luelmo 5 , Pantelis Afroudakis 1 , Kalliopi Georgikopoulou 1 , Paloma Tejera Nevado 6 , Julia Eick 6 , Eugenia Bifeld 6 , María J Corral 7 , María Dolores Jiménez-Antón 7 , Bernhard Ellinger 8 , Maria Kuzikov 8 , Irini Fragiadaki 9 , Effie Scoulica 9 , Sheraz Gul 8 , Joachim Clos 6 , Kyriakos C Prousis 1 , Juan J Torrado 10 , José María Alunda 7 , Rebecca C Wade 11 , Wanderley de Souza 3 , Anabela Cordeiro da Silva 12 , Theodora Calogeropoulou 1
|
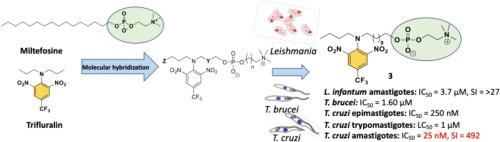
A series of nine novel ether phospholipid-dinitroaniline hybrids were synthesized in an effort to deliver more potent antiparasitic agents with improved safety profile compared to miltefosine. The compounds were evaluated for their in vitro antiparasitic activity against L. infantum, L.donovani, L. amazonensis, L. major and L. tropica promastigotes, L. infantum and L. donovani intracellular amastigotes, Trypanosoma brucei brucei and against different developmental stages of Trypanosoma cruzi. The nature of the oligomethylene spacer between the dinitroaniline moiety and the phosphate group, the length of the side chain substituent on the dinitroaniline and the choline or homocholine head group were found to affect both the activity and toxicity of the hybrids. The early ADMET profile of the derivatives did not reveal major liabilities. Hybrid 3, bearing an 11-carbon oligomethylene spacer, a butyl side chain and a choline head group, was the most potent analogue of the series. It exhibited a broad spectrum antiparasitic profile against the promastigotes of New and Old World Leishmania spp., against intracellular amastigotes of two L. infantum strains and L. donovani, against T. brucei and against T. cruzi Y strain epimastigotes, intracellular amastigotes and trypomastigotes. The early toxicity studies revealed that hybrid 3 showed a safe toxicological profile while its cytotoxicity concentration (CC50) against THP-1 macrophages being >100 μM. Computational analysis of binding sites and docking indicated that the interaction of hybrid 3 with trypanosomatid α-tubulin may contribute to its mechanism of action. Furthermore, compound 3 was found to interfere with the cell cycle in T. cruzi epimastigotes, while ultrastructural studies using SEM and TEM in T. cruzi showed that compound 3 affects cellular processes that result in changes in the Golgi complex, the mitochondria and the parasite’s plasma membrane. The snapshot pharmacokinetic studies showed low levels of 3 after 24 h following oral administration of 100 mg/Kg, while, its homocholine congener compound 9 presented a better pharmacokinetic profile.
中文翻译:

抗寄生虫二硝基苯胺醚磷脂杂化物的设计、合成和生物学评价
合成了一系列九种新型醚磷脂-二硝基苯胺杂化物,旨在提供更有效的抗寄生虫药,与米替福辛相比,安全性更高。评估了化合物对婴儿乳杆菌、多诺瓦尼乳杆菌、亚马逊乳杆菌、硕大乳杆菌和热带乳杆菌前鞭毛体、婴儿乳杆菌和多诺瓦尼乳杆菌细胞内无鞭毛体、布氏锥虫以及针对不同发育阶段的体外抗寄生虫活性。克氏锥虫。发现二硝基苯胺部分和磷酸基团之间的低聚亚甲基间隔基的性质、二硝基苯胺和胆碱或高胆碱头基上的侧链取代基的长度影响杂化物的活性和毒性。衍生品的早期 ADMET 概况并未揭示主要负债。Hybrid 3带有 11 个碳低聚亚甲基间隔基、丁基侧链和胆碱头基,是该系列中最有效的类似物。它对新世界利什曼原虫属和旧世界利什曼原虫属的前鞭毛体、两种婴儿利什曼原虫菌株和多诺瓦尼利什曼原虫的细胞内无鞭毛体、对布氏利什曼原虫和克氏利什曼原虫 Y 菌株的上鞭毛体、细胞内无鞭毛体和锥鞭毛体表现出广谱抗寄生虫特性。 。早期毒性研究表明,hybrid 3显示出安全的毒理学特征,同时其针对 THP-1 巨噬细胞的细胞毒性浓度 (CC 50 ) >100 μM。结合位点和对接的计算分析表明,hybrid 3与锥虫α-微管蛋白的相互作用可能有助于其作用机制。此外,发现化合物3会干扰克氏锥虫上鞭毛体的细胞周期,而使用 SEM 和 TEM 对克氏锥虫进行的超微结构研究表明,化合物3影响细胞过程,从而导致高尔基复合体、线粒体和寄生虫的变化。质膜。快照药代动力学研究显示,口服 100 mg/Kg 后 24 小时后3的水平较低,而其高胆碱同源化合物9则呈现出更好的药代动力学特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号