当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical Synthesis of Tenofovir Alafenamide Fumarate Inspired by New Retrosynthetic Disconnection Featuring a Novel Carbon–Phosphorus Bond Construction Methodology
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-05-19 , DOI: 10.1021/acs.oprd.3c00070 Xiuping Liu 1, 2 , Chen Chen 1 , Muzi Li 1, 2 , Man Li 1 , Jie Ren 1 , Qingwen Zhang 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-05-19 , DOI: 10.1021/acs.oprd.3c00070 Xiuping Liu 1, 2 , Chen Chen 1 , Muzi Li 1, 2 , Man Li 1 , Jie Ren 1 , Qingwen Zhang 1
Affiliation
|
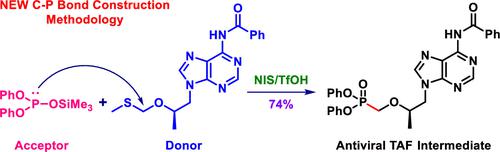
Tenofovir alafenamide fumarate (TAF) is rising as a mainstay antiretroviral agent for the treatment of HIV and chronic HBV infections. A de novo practical synthesis of TAF circumventing tenofovir (PMPA) has been accomplished on a 7 g scale. This reimagined synthesis of TAF, inspired by a hitherto uncharted retrosynthetic disconnection, centers on the P-alkylation of silylated diphenyl phosphonate 11 (as acceptor) with methylthiomethyl (MTM) ether derivative 12 (as donor) in the presence of NIS/TfOH combination as a promoter to construct the strategic carbon–phosphorus bond. This PMPA-free synthesis of TAF not only removes the intrinsic drawbacks encountered by the PMPA-dependent commercial process but also is beneficial to the diversification of the synthetic portfolio of TAF. Furthermore, this type of P-alkylation reaction with defined stereochemistry could be deployed for the late-stage modification of druglike molecules and natural products to access valuable phosphonate derivatives.
中文翻译:

受新型逆合成断裂启发的富马酸替诺福韦艾拉酚胺的实际合成,采用新型碳磷键构建方法,受新型逆合成断裂启发的富马酸替诺福韦艾拉酚胺的实际合成,采用新型碳磷键构建方法
富马酸替诺福韦艾拉酚胺 (TAF) 正在成为治疗 HIV 和慢性 HBV 感染的主要抗逆转录病毒药物。绕过 TAF 的替诺福韦 (PMPA) 的从头实用合成已在 7 g 规模上完成。TAF 的这种重新构想的合成,受到迄今为止未知的逆合成断开的启发,以甲硫基甲基 (MTM) 醚衍生物 12 对硅烷化二苯基膦酸酯 11(作为受体)的P-烷基化为中心(作为供体)在 NIS/TfOH 组合存在下作为促进剂构建战略碳磷键。这种不含 PMPA 的 TAF 合成不仅消除了依赖 PMPA 的商业工艺所遇到的固有缺陷,而且有利于 TAF 合成产品组合的多样化。此外,这种具有明确立体化学的P -烷基化反应可用于药物分子和天然产物的后期修饰,以获得有价值的膦酸酯衍生物。,富马酸替诺福韦艾拉酚胺 (TAF) 正在成为治疗 HIV 和慢性 HBV 感染的主要抗逆转录病毒药物。绕过 TAF 的替诺福韦 (PMPA) 的从头实用合成已在 7 g 规模上完成。TAF 的这种重新构想的合成,受到迄今为止未知的逆合成断开的启发,以甲硫基甲基 (MTM) 醚衍生物 12 对硅烷化二苯基膦酸酯 11(作为受体)的P-烷基化为中心(作为供体)在 NIS/TfOH 组合存在下作为促进剂构建战略碳磷键。这种不含 PMPA 的 TAF 合成不仅消除了依赖 PMPA 的商业工艺所遇到的固有缺陷,而且有利于 TAF 合成产品组合的多样化。此外,这种具有明确立体化学的P -烷基化反应可用于药物分子和天然产物的后期修饰,以获得有价值的膦酸酯衍生物。
更新日期:2023-05-19
中文翻译:

受新型逆合成断裂启发的富马酸替诺福韦艾拉酚胺的实际合成,采用新型碳磷键构建方法,受新型逆合成断裂启发的富马酸替诺福韦艾拉酚胺的实际合成,采用新型碳磷键构建方法
富马酸替诺福韦艾拉酚胺 (TAF) 正在成为治疗 HIV 和慢性 HBV 感染的主要抗逆转录病毒药物。绕过 TAF 的替诺福韦 (PMPA) 的从头实用合成已在 7 g 规模上完成。TAF 的这种重新构想的合成,受到迄今为止未知的逆合成断开的启发,以甲硫基甲基 (MTM) 醚衍生物 12 对硅烷化二苯基膦酸酯 11(作为受体)的P-烷基化为中心(作为供体)在 NIS/TfOH 组合存在下作为促进剂构建战略碳磷键。这种不含 PMPA 的 TAF 合成不仅消除了依赖 PMPA 的商业工艺所遇到的固有缺陷,而且有利于 TAF 合成产品组合的多样化。此外,这种具有明确立体化学的P -烷基化反应可用于药物分子和天然产物的后期修饰,以获得有价值的膦酸酯衍生物。,富马酸替诺福韦艾拉酚胺 (TAF) 正在成为治疗 HIV 和慢性 HBV 感染的主要抗逆转录病毒药物。绕过 TAF 的替诺福韦 (PMPA) 的从头实用合成已在 7 g 规模上完成。TAF 的这种重新构想的合成,受到迄今为止未知的逆合成断开的启发,以甲硫基甲基 (MTM) 醚衍生物 12 对硅烷化二苯基膦酸酯 11(作为受体)的P-烷基化为中心(作为供体)在 NIS/TfOH 组合存在下作为促进剂构建战略碳磷键。这种不含 PMPA 的 TAF 合成不仅消除了依赖 PMPA 的商业工艺所遇到的固有缺陷,而且有利于 TAF 合成产品组合的多样化。此外,这种具有明确立体化学的P -烷基化反应可用于药物分子和天然产物的后期修饰,以获得有价值的膦酸酯衍生物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号