Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2023-05-19 , DOI: 10.1016/j.jechem.2023.04.040
Ziyu Zhou , Shuyu Liang , Jiewen Xiao , Tianyu Zhang , Min Li , Wenfu Xie , Qiang Wang
|
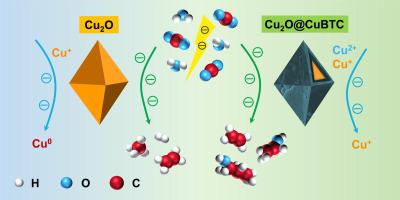
Copper (Cu)-based materials are known as the most attractive catalysts for electrochemical carbon dioxide reduction reaction (CO2RR), especially the Cu+ species (e.g., Cu2O), which show excellent capability for catalyzing CO2 to C2+ chemicals because of their unique electronic structure. However, the active Cu+ species are prone to be reduced to metallic Cu under an electroreduction environment, thus resulting in fast deactivation and poor selectivity. Here, we developed an advanced surface modification strategy to maintain the active Cu+ species via assembling a protective layer of metal–organic framework (copper benzenetricarboxylate, CuBTC) on the surface of Cu2O octahedron (Cu2O@CuBTC). It's encouraging to see that the Cu2O@CuBTC heterostructure outperforms the bare Cu2O octahedron in catalyzing CO2 to C2+ chemicals and dramatically enhances the ratio of C2H4/CH4 products. A systematic study reveals that the introduced CuBTC shell plays a critical role in maintaining the active Cu+ species in Cu2O@CuBTC heterostructure under reductive conditions. This work offers a practical strategy for improving the catalytic performance of CO2RR over copper oxides and also establishes a route to maintain the state of valence-sensitive catalysts.
中文翻译:

使用稳定的 Cu+ 对 Cu2O 进行表面修饰,以高效稳定的 CO2 电还原为 C2+ 化学品
铜(Cu)基材料被认为是电化学二氧化碳还原反应(CO 2 RR)中最有吸引力的催化剂,尤其是Cu +物质(例如Cu 2 O),其表现出优异的催化CO 2到C 2的能力+化学品因其独特的电子结构。然而,活性Cu +物种在电还原环境下容易被还原为金属Cu,导致失活速度快且选择性差。在这里,我们开发了一种先进的表面改性策略来保持活性 Cu +通过在Cu 2 O八面体(Cu 2 O@ CuBTC)表面组装金属有机骨架保护层(苯三甲酸铜,CuBTC)来形成物种。令人鼓舞的是,Cu 2 O@CuBTC异质结构在催化CO 2生成C 2+化学品方面优于裸露的Cu 2 O八面体,并显着提高了C 2 H 4 /CH 4产物的比率。系统研究表明,引入的CuBTC壳对于维持Cu 2中的活性Cu +物种起着关键作用还原条件下的O@CuBTC异质结构。这项工作为提高CO 2 RR相对于铜氧化物的催化性能提供了实用策略,并建立了维持价态敏感催化剂状态的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号