当前位置:
X-MOL 学术
›
Chin. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multi-electron reaction and fast Al ion diffusion of δ-MnO2 cathode materials in rechargeable aluminum batteries via first-principle calculations
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-05-19 , DOI: 10.1016/j.cclet.2023.108589 Lumin Zheng , Ying Bai , Chuan Wu
Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-05-19 , DOI: 10.1016/j.cclet.2023.108589 Lumin Zheng , Ying Bai , Chuan Wu
|
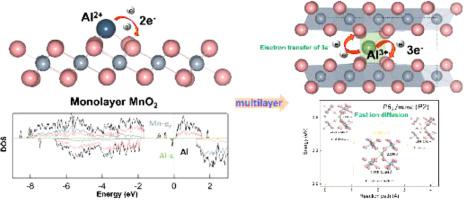
Rechargeable aluminum batteries with multi-electron reaction have a high theoretical capacity for next generation of energy storage devices. However, the diffusion mechanism and intrinsic property of Al insertion into MnO are not clear. Hence, based on the first-principles calculations, key influencing factors of slow Al-ions diffusion are narrow pathways, unstable Al-O bonds and Mn type polaron have been identified by investigating four types of -MnO (O3, O’3, P2 and T1). Although Al insert into -MnO leads to a decrease in the spacing of the Mn-Mn layer, P2 type MnO keeps the long (spacious pathways) and stable (2.007–2.030 Å) Al-O bonds resulting in the lower energy barrier of Al diffusion of 0.56 eV. By eliminated the influence of Mn (low concentration of Al insertion), the energy barrier of Al migration achieves 0.19 eV in P2 type, confirming the obviously effect of Mn polaron. On the contrary, although the T1 type MnO has the sluggish of Al-ions diffusion, the larger interlayer spacing of Mn-Mn layer, causing by HO could assist Al-ions diffusion. Furthermore, it is worth to notice that the multilayer -MnO achieves multi-electron reaction of 3|e|. Considering the requirement of high energy density, the average voltage of P2 (1.76 V) is not an obstacle for application as cathode in RABs. These discover suggest that layered MnO should keep more P2-type structure in the synthesis of materials and increase the interlayer spacing of Mn-Mn layer for providing technical support of RABs in large-scale energy storage.
中文翻译:

通过第一性原理计算可充电铝电池中δ-MnO2正极材料的多电子反应和快速Al离子扩散
具有多电子反应的可充电铝电池对于下一代储能装置具有很高的理论容量。然而,Al插入MnO的扩散机制和固有性质尚不清楚。因此,基于第一性原理计算,通过研究四种类型的-MnO(O3、O'3、P2),确定了缓慢Al离子扩散的关键影响因素是狭窄的通道、不稳定的Al-O键和Mn型极化子。和T1)。尽管Al插入-MnO中导致Mn-Mn层间距减小,但P2型MnO保持了长(宽敞的路径)和稳定的(2.007–2.030 Å)Al-O键,导致Al的能垒较低0.56 eV 的扩散。通过消除Mn(低浓度Al插入)的影响,P2型Al迁移的能垒达到0.19 eV,证实了Mn极化子的明显作用。相反,虽然T1型MnO的Al离子扩散较慢,但H2O引起的Mn-Mn层的层间距较大,有利于Al离子的扩散。此外,值得注意的是,多层-MnO实现了3|e|的多电子反应。考虑到高能量密度的要求,P2的平均电压(1.76 V)对于作为RAB阴极的应用并不构成障碍。这些发现表明,层状MnO在材料合成中应保留更多的P2型结构,并增加Mn-Mn层的层间距,为RABs大规模储能提供技术支撑。
更新日期:2023-05-19
中文翻译:

通过第一性原理计算可充电铝电池中δ-MnO2正极材料的多电子反应和快速Al离子扩散
具有多电子反应的可充电铝电池对于下一代储能装置具有很高的理论容量。然而,Al插入MnO的扩散机制和固有性质尚不清楚。因此,基于第一性原理计算,通过研究四种类型的-MnO(O3、O'3、P2),确定了缓慢Al离子扩散的关键影响因素是狭窄的通道、不稳定的Al-O键和Mn型极化子。和T1)。尽管Al插入-MnO中导致Mn-Mn层间距减小,但P2型MnO保持了长(宽敞的路径)和稳定的(2.007–2.030 Å)Al-O键,导致Al的能垒较低0.56 eV 的扩散。通过消除Mn(低浓度Al插入)的影响,P2型Al迁移的能垒达到0.19 eV,证实了Mn极化子的明显作用。相反,虽然T1型MnO的Al离子扩散较慢,但H2O引起的Mn-Mn层的层间距较大,有利于Al离子的扩散。此外,值得注意的是,多层-MnO实现了3|e|的多电子反应。考虑到高能量密度的要求,P2的平均电压(1.76 V)对于作为RAB阴极的应用并不构成障碍。这些发现表明,层状MnO在材料合成中应保留更多的P2型结构,并增加Mn-Mn层的层间距,为RABs大规模储能提供技术支撑。






























 京公网安备 11010802027423号
京公网安备 11010802027423号