当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Recognition Studies of Thioamide (−CSNH2) Functionality through Co-crystals of Some Thiobenzamides with N-Donor Ligands: Evaluation and Correlation of Structural Landscapes with Morphology and Lattice Energy
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-05-19 , DOI: 10.1021/acs.cgd.3c00350
Sonali Ghosh 1 , Venkateswara Rao Pedireddi 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2023-05-19 , DOI: 10.1021/acs.cgd.3c00350
Sonali Ghosh 1 , Venkateswara Rao Pedireddi 1
Affiliation
|
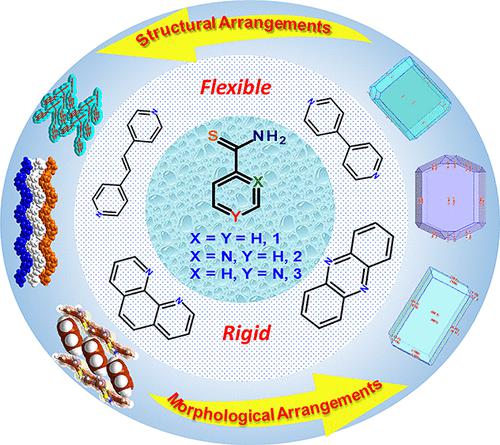
Molecular recognition studies of supramolecular edifices of thiobenzamide, 1, and its hetero analogues, o- and p-isomers of pyridinethioamides, 2 and 3, respectively, have been reported highlighting the recognition patterns of the thioamides with different aza-donor compounds having variable conformational flexibility and dimensions, for example, 4,4′-bipyridine (bpy, a), 1,2-bis(4-pyridyl)ethene (bpyee, b), 1,10-phenanthroline (110phen, c), and phenazine (phenz, d). The analysis reveals that thioamides 1–3 produce co-crystals, 1(a–d)−3(a–d), respectively, with the corresponding aza-donors. These structures, determined by single-crystal X-ray diffraction, reveal that in all co-crystals, the aggregation of the coformers occurs through N–H···N hydrogen bonds due to the establishment of recognition between the thioamide moiety and the N-hetero atom and is further augmented by C–H···S hydrogen bonds. Analysis of hydrogen-bonding patterns reveals several structural similarities and differences among co-crystals, with the formation of different types of three-dimensional structures in the form of herringbone, layer, and sandwich. A systematic evaluation of solid-state structures was performed in terms of the interplay of competing intermolecular interactions within the crystal lattices and packing features of the resulted exotic architectures, BFDH morphology predictions, and isostructural analysis through cell-similarity index, powder X-ray diffraction similarity, etc. Furthermore, the importance of each type of intermolecular interaction, in particular, N–H···N, N–H···S, C–H···S, etc., has been quantified by Hirshfeld surface analysis. In addition, the energy contribution of all interactions is computed by developing energy frameworks using Crystal Explorer.
中文翻译:

通过一些硫代苯甲酰胺与 N-供体配体的共晶对硫代酰胺 (-CSNH2) 功能的分子识别研究:结构景观与形态学和晶格能量的评估和相关性
硫代苯甲酰胺1及其异类类似物,吡啶硫代酰胺2和3的邻异构体和对异构体的超分子结构的分子识别研究已被报道,突出了硫代酰胺与具有可变构象的不同氮杂供体化合物的识别模式柔韧性和尺寸,例如,4,4'-联吡啶 ( bpy , a )、1,2-双(4-吡啶基)乙烯 ( bpyee , b )、1,10-菲咯啉 ( 110phen , c ) 和吩嗪 (苯, d ). 分析表明,硫代酰胺1–3产生共晶,1 ( a – d ) −3 ( a – d ),分别与相应的氮杂供体。这些由单晶 X 射线衍射确定的结构表明,在所有共晶中,由于硫代酰胺部分与N-杂原子,并被C-H···S氢键进一步增强。对氢键模式的分析揭示了共晶之间的几种结构相似性和差异性,形成了人字形、层状和三明治形式的不同类型的三维结构。根据晶格内竞争性分子间相互作用的相互作用和所得奇异结构的堆积特征、BFDH 形态预测以及通过细胞相似指数、粉末 X 射线衍射进行的同构分析,对固态结构进行了系统评估相似性等。此外,Hirshfeld 已经量化了每种分子间相互作用的重要性,特别是 N–H···N、N–H···S、C–H···S 等表面分析。此外,
更新日期:2023-05-19
中文翻译:

通过一些硫代苯甲酰胺与 N-供体配体的共晶对硫代酰胺 (-CSNH2) 功能的分子识别研究:结构景观与形态学和晶格能量的评估和相关性
硫代苯甲酰胺1及其异类类似物,吡啶硫代酰胺2和3的邻异构体和对异构体的超分子结构的分子识别研究已被报道,突出了硫代酰胺与具有可变构象的不同氮杂供体化合物的识别模式柔韧性和尺寸,例如,4,4'-联吡啶 ( bpy , a )、1,2-双(4-吡啶基)乙烯 ( bpyee , b )、1,10-菲咯啉 ( 110phen , c ) 和吩嗪 (苯, d ). 分析表明,硫代酰胺1–3产生共晶,1 ( a – d ) −3 ( a – d ),分别与相应的氮杂供体。这些由单晶 X 射线衍射确定的结构表明,在所有共晶中,由于硫代酰胺部分与N-杂原子,并被C-H···S氢键进一步增强。对氢键模式的分析揭示了共晶之间的几种结构相似性和差异性,形成了人字形、层状和三明治形式的不同类型的三维结构。根据晶格内竞争性分子间相互作用的相互作用和所得奇异结构的堆积特征、BFDH 形态预测以及通过细胞相似指数、粉末 X 射线衍射进行的同构分析,对固态结构进行了系统评估相似性等。此外,Hirshfeld 已经量化了每种分子间相互作用的重要性,特别是 N–H···N、N–H···S、C–H···S 等表面分析。此外,

































 京公网安备 11010802027423号
京公网安备 11010802027423号