当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of 4-(4-aminophenoxy)picolinamide derivatives as potential antitumor agents
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-05-18 , DOI: 10.1016/j.ejmech.2023.115499
Jintian Dai 1 , Jianqing Zhang 2 , Dongxue Fu 3 , Meng Liu 4 , Han Zhang 4 , Sheng Tang 4 , Linxiao Wang 4 , Shan Xu 4 , Wufu Zhu 4 , Qidong Tang 5 , Pengwu Zheng 4 , Ting Chen 3
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-05-18 , DOI: 10.1016/j.ejmech.2023.115499
Jintian Dai 1 , Jianqing Zhang 2 , Dongxue Fu 3 , Meng Liu 4 , Han Zhang 4 , Sheng Tang 4 , Linxiao Wang 4 , Shan Xu 4 , Wufu Zhu 4 , Qidong Tang 5 , Pengwu Zheng 4 , Ting Chen 3
Affiliation
|
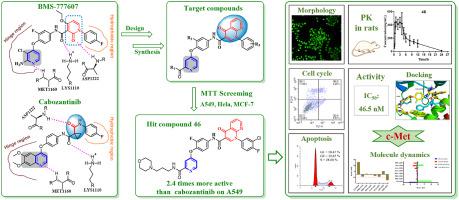
Cancer is a leading cause of death in humans. Molecular targeted therapy for cancer has become a research hotspot as it is associated with low toxicity and high efficiency. In this study, a total of 36 derivatives of 4-(4-aminophenoxy)pyridinamide were designed and synthesized, based on the analysis of the binding patterns of cabozantinib and BMS-777607 to MET protein. Most target compounds exhibited moderate to excellent antiproliferative activity against three different cell lines (A549, HeLa and MCF-7). A total of 7 compounds had stronger inhibitory activities than cabozantinib, and the IC value of the most promising compound was 0.26 μM against the A549 cells, which was 2.4 times more active than that of cabozantinib. The structure-activity relationship of the target compounds was analyzed and summarized, and the action mechanism was discussed. The acridine orange (AO) staining assay and cell cycle apoptosis revealed that compound dose-dependently induced apoptosis of A549 cells, and blocked the cells mainly in G0/G1 phase. The IC value of compound on c-Met kinase was 46.5 nM. Further docking studies and molecular dynamics simulations signaled that compound formed four key hydrogen bonds to c-Met kinase, and these key amino acids played a major role in binding free energy. In addition, compound also showed good pharmacokinetic characteristics in rats. In conclusion, compound is a promising antitumor agent.
中文翻译:

4-(4-氨基苯氧基)吡啶酰胺衍生物作为潜在抗肿瘤药物的设计、合成和生物学评价
癌症是人类死亡的主要原因。癌症分子靶向治疗因其低毒、高效而成为研究热点。本研究基于卡博替尼和BMS-777607与MET蛋白的结合模式分析,设计并合成了总共36种4-(4-氨基苯氧基)吡啶酰胺衍生物。大多数目标化合物对三种不同的细胞系(A549、HeLa 和 MCF-7)表现出中等至优异的抗增殖活性。共有7种化合物比卡博替尼具有更强的抑制活性,其中最有前景的化合物对A549细胞的IC50值为0.26 μM,是卡博替尼活性的2.4倍。对目标化合物的构效关系进行了分析和总结,并探讨了作用机制。吖啶橙(AO)染色实验和细胞周期凋亡分析表明,化合物剂量依赖性地诱导A549细胞凋亡,并主要将细胞阻滞于G0/G1期。化合物对 c-Met 激酶的 IC 值为 46.5 nM。进一步的对接研究和分子动力学模拟表明,该化合物与c-Met激酶形成了四个关键氢键,这些关键氨基酸在结合自由能方面发挥了重要作用。此外,化合物在大鼠体内也表现出良好的药代动力学特征。总之,该化合物是一种有前途的抗肿瘤剂。
更新日期:2023-05-18
中文翻译:

4-(4-氨基苯氧基)吡啶酰胺衍生物作为潜在抗肿瘤药物的设计、合成和生物学评价
癌症是人类死亡的主要原因。癌症分子靶向治疗因其低毒、高效而成为研究热点。本研究基于卡博替尼和BMS-777607与MET蛋白的结合模式分析,设计并合成了总共36种4-(4-氨基苯氧基)吡啶酰胺衍生物。大多数目标化合物对三种不同的细胞系(A549、HeLa 和 MCF-7)表现出中等至优异的抗增殖活性。共有7种化合物比卡博替尼具有更强的抑制活性,其中最有前景的化合物对A549细胞的IC50值为0.26 μM,是卡博替尼活性的2.4倍。对目标化合物的构效关系进行了分析和总结,并探讨了作用机制。吖啶橙(AO)染色实验和细胞周期凋亡分析表明,化合物剂量依赖性地诱导A549细胞凋亡,并主要将细胞阻滞于G0/G1期。化合物对 c-Met 激酶的 IC 值为 46.5 nM。进一步的对接研究和分子动力学模拟表明,该化合物与c-Met激酶形成了四个关键氢键,这些关键氨基酸在结合自由能方面发挥了重要作用。此外,化合物在大鼠体内也表现出良好的药代动力学特征。总之,该化合物是一种有前途的抗肿瘤剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号