当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Circumventing CO2 Reduction Scaling Relations Over the Heteronuclear Diatomic Catalytic Pair
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-18 , DOI: 10.1021/jacs.3c03426 Jie Ding 1, 2 , Fuhua Li 2 , Jincheng Zhang 2 , Qiao Zhang 1 , Yuhang Liu 3 , Weijue Wang 4 , Wei Liu 4 , Beibei Wang 5 , Jun Cai 5 , Xiaozhi Su 3 , Hong Bin Yang 4 , Xuan Yang 6 , Yanqiang Huang 4 , Yueming Zhai 1 , Bin Liu 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-18 , DOI: 10.1021/jacs.3c03426 Jie Ding 1, 2 , Fuhua Li 2 , Jincheng Zhang 2 , Qiao Zhang 1 , Yuhang Liu 3 , Weijue Wang 4 , Wei Liu 4 , Beibei Wang 5 , Jun Cai 5 , Xiaozhi Su 3 , Hong Bin Yang 4 , Xuan Yang 6 , Yanqiang Huang 4 , Yueming Zhai 1 , Bin Liu 2
Affiliation
|
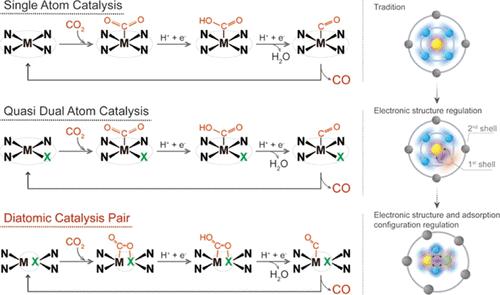
In the electrochemical CO2 reduction reaction (CO2RR), CO2 activation is always the first step, followed by the subsequent hydrogenation. The catalytic performance of CO2RR is intrinsically restricted by the competition between molecular CO2 activation and CO2 reduction product release. Here, we design a heteronuclear Fe1-Mo1 dual-metal catalytic pair on ordered porous carbon that features a high catalytic performance for driving electrochemical CO2 reduction to CO. Combining real-time near-ambient pressure X-ray photoelectron spectroscopy, operando 57Fe Mössbauer spectroscopy, and in situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy measurements with density functional theory calculations, chemical adsorption of CO2 is observed on the Fe1-Mo1 catalytic pair through a bridge configuration, which prompts the bending of the CO2 molecule for CO2 activation and then facilitates the subsequent hydrogeneration reaction. More importantly, the dynamic adsorption configuration transition from the bridge configuration of CO2 on Fe1-Mo1 to the linear configuration of CO on the Fe1 center results in breaking the scaling relationship in CO2RR, simultaneously promoting the CO2 activation and the CO release.
中文翻译:

绕过异核双原子催化对的 CO2 还原比例关系
在电化学CO 2还原反应(CO 2 RR)中,CO 2活化始终是第一步,随后是随后的氢化反应。CO 2 RR的催化性能本质上受到分子CO 2活化和CO 2还原产物释放之间竞争的限制。在这里,我们在有序多孔碳上设计了异核 Fe 1 -Mo 1双金属催化对,其具有高催化性能,可驱动电化学 CO 2还原为 CO。结合实时近环境压力 X 射线光电子能谱、操作数57Fe Mössbauer 光谱、原位衰减全反射表面增强红外吸收光谱测量和密度泛函理论计算,通过桥构型在 Fe 1 -Mo 1催化对上观察到CO 2的化学吸附,这促使 CO 2 的弯曲CO 2分子用于CO 2活化,进而促进后续的加氢反应。更重要的是,动态吸附构型从 CO 2在 Fe 1 -Mo 1上的桥接构型转变为 CO 在 Fe 1上的线性构型中心结果打破了 CO 2 RR中的标度关系,同时促进了 CO 2活化和 CO 释放。
更新日期:2023-05-18
中文翻译:

绕过异核双原子催化对的 CO2 还原比例关系
在电化学CO 2还原反应(CO 2 RR)中,CO 2活化始终是第一步,随后是随后的氢化反应。CO 2 RR的催化性能本质上受到分子CO 2活化和CO 2还原产物释放之间竞争的限制。在这里,我们在有序多孔碳上设计了异核 Fe 1 -Mo 1双金属催化对,其具有高催化性能,可驱动电化学 CO 2还原为 CO。结合实时近环境压力 X 射线光电子能谱、操作数57Fe Mössbauer 光谱、原位衰减全反射表面增强红外吸收光谱测量和密度泛函理论计算,通过桥构型在 Fe 1 -Mo 1催化对上观察到CO 2的化学吸附,这促使 CO 2 的弯曲CO 2分子用于CO 2活化,进而促进后续的加氢反应。更重要的是,动态吸附构型从 CO 2在 Fe 1 -Mo 1上的桥接构型转变为 CO 在 Fe 1上的线性构型中心结果打破了 CO 2 RR中的标度关系,同时促进了 CO 2活化和 CO 释放。

































 京公网安备 11010802027423号
京公网安备 11010802027423号