当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-trifluoromethyl benzimidazoles, benzoxazoles, and benzothiazoles via condensation of diamines or amino(thio)phenols with CF3CN
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-05-18 , DOI: 10.1039/d3ob00517h Bo Lin 1 , Yunfei Yao 1 , Minze Wu 2 , Lu Qin 2 , Shouxiong Chen 2 , Yi You 1 , Zhiqiang Weng 1, 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-05-18 , DOI: 10.1039/d3ob00517h Bo Lin 1 , Yunfei Yao 1 , Minze Wu 2 , Lu Qin 2 , Shouxiong Chen 2 , Yi You 1 , Zhiqiang Weng 1, 2
Affiliation
|
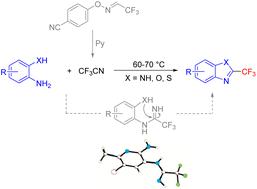
A new and efficient method is developed for the synthesis of 2-trifluoromethyl benzimidazoles, benzoxazoles, and benzothiazoles in good to excellent yields by the condensation of diamines or amino(thio)phenols with in situ generated CF3CN. Additionally, the synthetic utility of the 2-trifluoromethyl benzimidazole and benzoxazole products is demonstrated via gram scale synthesis. The mechanistic study suggests that the reaction proceeds via the nucleophilic addition of trifluoroacetonitrile to the amino group of the diamine derivatives to form an imidamide intermediate, followed by intramolecular cyclization.
中文翻译:

二胺或氨基(硫代)酚与 CF3CN 缩合合成 2-三氟甲基苯并咪唑、苯并恶唑和苯并噻唑
通过二胺或氨基(硫代)酚与原位生成的 CF 3 CN 的缩合,开发了一种新的有效方法,以良好至极好的收率合成 2-三氟甲基苯并咪唑、苯并恶唑和苯并噻唑。此外,通过克级合成证明了 2-三氟甲基苯并咪唑和苯并恶唑产品的合成效用。机理研究表明,该反应通过三氟乙腈与二胺衍生物的氨基进行亲核加成反应,形成亚胺中间体,然后进行分子内环化。
更新日期:2023-05-18
中文翻译:

二胺或氨基(硫代)酚与 CF3CN 缩合合成 2-三氟甲基苯并咪唑、苯并恶唑和苯并噻唑
通过二胺或氨基(硫代)酚与原位生成的 CF 3 CN 的缩合,开发了一种新的有效方法,以良好至极好的收率合成 2-三氟甲基苯并咪唑、苯并恶唑和苯并噻唑。此外,通过克级合成证明了 2-三氟甲基苯并咪唑和苯并恶唑产品的合成效用。机理研究表明,该反应通过三氟乙腈与二胺衍生物的氨基进行亲核加成反应,形成亚胺中间体,然后进行分子内环化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号