当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Self-Assembly Supramolecular Antagonist Reinforces Radiotherapy by Inhibiting Tumor Apoptosis Evasion
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-05-17 , DOI: 10.1002/adfm.202302697 Xiaoxue Hou 1 , Lijun Yang 1 , Paiyun Li 2 , Jinjian Liu 1 , Yumin Zhang 1 , Qian Wang 1 , Dianyu Wang 1 , Shiyu Peng 1 , Linzhu Su 1 , Wenxue Zhang 2 , Fan Huang 1 , Jianfeng Liu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-05-17 , DOI: 10.1002/adfm.202302697 Xiaoxue Hou 1 , Lijun Yang 1 , Paiyun Li 2 , Jinjian Liu 1 , Yumin Zhang 1 , Qian Wang 1 , Dianyu Wang 1 , Shiyu Peng 1 , Linzhu Su 1 , Wenxue Zhang 2 , Fan Huang 1 , Jianfeng Liu 1
Affiliation
|
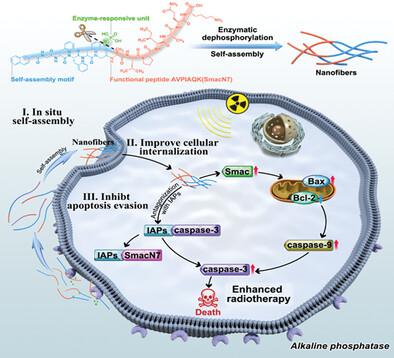
Radiosensitizers hold great promise for enhanced cancer radiotherapeutics. However, apoptosis evasion of cancerous cells usually limits the efficiency of radiosensitive strategies. Herein, an in situ self-assembled supramolecular antagonist is developed to reinforce the treatment outcome of radiotherapy by inhibiting tumor apoptosis evasion. The supramolecular antagonist is composed of self-assembled peptide functionalized with apoptosis-inducing peptide SmacN7 and alkaline phosphatase (ALP)-responsive group. Upon reaching the tumor site, the supramolecular antagonist can in situ form membrane-localized nanofibers triggered by ALP overexpressing in tumor cells, leading to enhanced cellular internalization. As a result, the cell-permeable supramolecular antagonist effectively binds to the inhibitor of apoptosis proteins (IAPs) and eliminates their inhibitory effect on caspase activity, thereby remarkably blocking the apoptosis evasion of tumor cells and boosting the therapeutic efficacy of radiotherapy. Furthermore, in vivo studies confirm that treatment with in situ self-assembled supramolecular antagonists can enhance radiation-induced tumor destruction without perceptible systemic toxicity. This study offers a novel strategy of tumor apoptosis evasion inhibition to potentiate radiotherapy, which may be instructive to the development of advanced cancer therapies.
中文翻译:

原位自组装超分子拮抗剂通过抑制肿瘤细胞凋亡逃避来增强放射治疗
放射增敏剂在增强癌症放射治疗方面具有广阔的前景。然而,癌细胞的凋亡逃避通常限制了放射敏感策略的效率。在此,开发了一种原位自组装超分子拮抗剂,通过抑制肿瘤细胞凋亡逃避来增强放疗的治疗效果。该超分子拮抗剂由凋亡诱导肽 SmacN7 和碱性磷酸酶 (ALP) 反应基团功能化的自组装肽组成。到达肿瘤部位后,超分子拮抗剂可以在肿瘤细胞中过度表达 ALP 的情况下原位形成膜定位的纳米纤维,从而增强细胞内化。因此,该细胞渗透性超分子拮抗剂与凋亡蛋白抑制剂(IAP)有效结合,消除其对Caspase活性的抑制作用,从而显着阻断肿瘤细胞的凋亡逃避,增强放疗的疗效。此外,体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。
更新日期:2023-05-17
中文翻译:

原位自组装超分子拮抗剂通过抑制肿瘤细胞凋亡逃避来增强放射治疗
放射增敏剂在增强癌症放射治疗方面具有广阔的前景。然而,癌细胞的凋亡逃避通常限制了放射敏感策略的效率。在此,开发了一种原位自组装超分子拮抗剂,通过抑制肿瘤细胞凋亡逃避来增强放疗的治疗效果。该超分子拮抗剂由凋亡诱导肽 SmacN7 和碱性磷酸酶 (ALP) 反应基团功能化的自组装肽组成。到达肿瘤部位后,超分子拮抗剂可以在肿瘤细胞中过度表达 ALP 的情况下原位形成膜定位的纳米纤维,从而增强细胞内化。因此,该细胞渗透性超分子拮抗剂与凋亡蛋白抑制剂(IAP)有效结合,消除其对Caspase活性的抑制作用,从而显着阻断肿瘤细胞的凋亡逃避,增强放疗的疗效。此外,体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。体内研究证实,用原位自组装超分子拮抗剂治疗可以增强辐射诱导的肿瘤破坏,而不会产生可察觉的全身毒性。这项研究提供了一种通过抑制肿瘤细胞凋亡逃避来增强放射治疗的新策略,这可能对先进癌症疗法的发展具有指导意义。













































 京公网安备 11010802027423号
京公网安备 11010802027423号