Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2023-05-16 , DOI: 10.1016/j.jcis.2023.05.072 Rui Wang 1 , Liyang Liu 1 , Shuhan Huang 1 , Yuheng Wu 1 , Xianghong Chen 1 , Zhiyong Liang 2 , Jiantie Xu 3
|
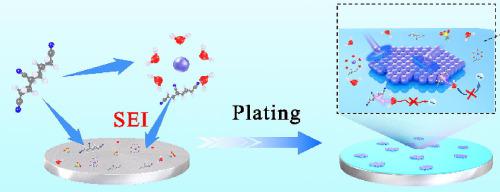
The growth of Zn dendrites and parasitic side reactions between electrode and electrolyte are major obstacles to the development of rechargeable aqueous zinc-ion batteries. To address these critical issues, the use of nitrile organic compounds as electrolyte additives holds great promise. Herein, for the first time, we prepared a small volume concentration (x) of 1,3,6-Hexanetricarbonitrile (HTCN-x) as additives into zinc trifluoromethanesulphonate (Zn(OTF)2) electrolyte and studied their electrochemical properties in Zn||ZnxV2O5·nH2O (Zn||ZVO) cells. It was found that the strong interaction between H2O and HTCN could significantly reduce the population of solvated H2O outside the solvation sheath, leading to reduced side reactions in the aqueous Zn(OTF)2 electrolyte. Moreover, the HTCN additive also facilitates the formation of strong and stable solid electrolyte interphase (SEI) film on the surface of the Zn anode, which effectively prevents the growth of Zn dendrites and the anode corrosion caused by the electrolyte. As a result, the HTCN-x (x = 0.3) electrolyte enabled the symmetrical Zn||Zn cell to cycle over 900 h at a current of 1 mA cm−2 with a limited capacity of 1 mAh cm−2. When the HTCN-0.3 electrolyte was used in Zn||ZVO cell, the cell delivered a high initial capacity of 355.6 mAh g−1 at 0.1 A g−1 and maintained a high capacity of 330.0 mAh g−1 at 1 A g−1 after 465 cycles.
中文翻译:

用于高性能水系锌离子电池的 1,3,6-己烷三腈的高效电解质添加剂
锌枝晶的生长和电极与电解质之间的寄生副反应是发展可充电水系锌离子电池的主要障碍。为了解决这些关键问题,使用腈类有机化合物作为电解质添加剂具有广阔的前景。在此,我们首次制备了小体积浓度 ( x ) 的 1,3,6-己烷三腈 (HTCN- x ) 作为添加剂加入三氟甲磺酸锌 (Zn(OTF) 2 ) 电解质中,并研究了它们在 Zn |中的电化学性质。 | Zn x V 2 O 5 ·nH 2 O (Zn || ZVO) 电池。发现 H 之间的强相互作用2 O 和 HTCN 可以显着减少溶剂化鞘外的溶剂化 H 2 O的数量,从而减少水性 Zn(OTF) 2电解质中的副反应。此外,HTCN添加剂还有助于在锌负极表面形成坚固稳定的固体电解质界面(SEI)膜,有效防止锌枝晶的生长和电解液引起的负极腐蚀。结果,HTCN- x ( x = 0.3) 电解质使对称的 Zn ||成为可能。锌电池以 1 mA cm -2的电流循环超过 900 小时,容量有限为 1 mAh cm -2。当HTCN-0.3电解液用于Zn|| ZVO 电池,该电池在 0.1 A g -1时提供 355.6 mAh g -1的高初始容量,并在 465 次循环后在 1 A g -1时保持 330.0 mAh g -1的高容量。

































 京公网安备 11010802027423号
京公网安备 11010802027423号