当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Synthesis of 3-Aryl-9H-imidazo[1,5-a]indol-9-ones Using Oxygen as the Sole Oxidant
Organic Letters ( IF 4.9 ) Pub Date : 2023-05-15 , DOI: 10.1021/acs.orglett.3c01148 Yufeng Chen 1 , Ruitong Yang 1 , Fuhong Xiao 1 , Tianci Xu 1 , Guojiang Mao 2 , Guo-Jun Deng 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-05-15 , DOI: 10.1021/acs.orglett.3c01148 Yufeng Chen 1 , Ruitong Yang 1 , Fuhong Xiao 1 , Tianci Xu 1 , Guojiang Mao 2 , Guo-Jun Deng 1
Affiliation
|
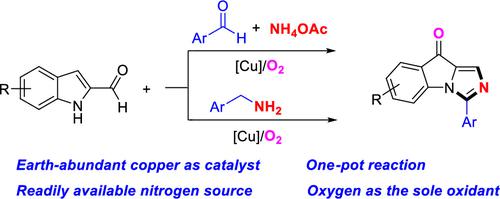
A three-component strategy was developed for 3-phenyl-9H-imidazo[1,5-a]indol-9-one preparation from indole-2-carboxaldehydes, aromatic aldehydes, and ammonium acetate under copper catalysis conditions. In this process, a new five-membered ring was formed and the C3 position in the indole substrate was selectively oxidized into a ketone skeleton using oxygen as the sole oxidant and ammonium acetate as the nitrogen source. Furthermore, same products also could be achieved from indole-2-carboxaldehydes and benzyl amines under similar reaction conditions.
中文翻译:

以氧为唯一氧化剂铜催化合成 3-Aryl-9H-imidazo[1,5-a]indol-9-ones
开发了一种三组分策略,用于在铜催化条件下从吲哚-2-甲醛、芳香醛和乙酸铵制备 3-苯基-9 H-咪唑并 [1,5- a ] 吲哚-9-酮。在此过程中,以氧为唯一氧化剂,以乙酸铵为氮源,形成一个新的五元环,吲哚底物的C3位被选择性氧化成酮骨架。此外,在类似的反应条件下,也可以从吲哚-2-甲醛和苄胺获得相同的产品。
更新日期:2023-05-15
中文翻译:

以氧为唯一氧化剂铜催化合成 3-Aryl-9H-imidazo[1,5-a]indol-9-ones
开发了一种三组分策略,用于在铜催化条件下从吲哚-2-甲醛、芳香醛和乙酸铵制备 3-苯基-9 H-咪唑并 [1,5- a ] 吲哚-9-酮。在此过程中,以氧为唯一氧化剂,以乙酸铵为氮源,形成一个新的五元环,吲哚底物的C3位被选择性氧化成酮骨架。此外,在类似的反应条件下,也可以从吲哚-2-甲醛和苄胺获得相同的产品。





































 京公网安备 11010802027423号
京公网安备 11010802027423号