当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO-Mediated Deactivation Mechanism of SiO2-Supported Copper Catalysts during Dimethyl Oxalate Hydrogenation to Ethylene Glycol
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-06-09 00:00:00 , DOI: 10.1021/acs.jpcc.5b03569 Jianwei Zheng 1 , Junfu Zhou 1 , Haiqiang Lin 1 , Xinping Duan 1 , Christopher T. Williams 2 , Youzhu Yuan 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-06-09 00:00:00 , DOI: 10.1021/acs.jpcc.5b03569 Jianwei Zheng 1 , Junfu Zhou 1 , Haiqiang Lin 1 , Xinping Duan 1 , Christopher T. Williams 2 , Youzhu Yuan 1
Affiliation
|
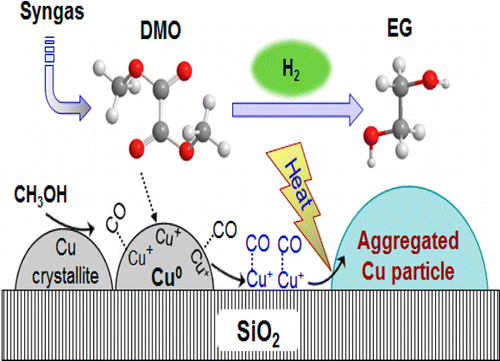
Selective hydrogenation of dimethyl oxalate (DMO) derived from syngas to ethylene glycol (EG) over copper (Cu)-based catalysts is an important transformation of modern syngas chemical industry. Methanol, as a product or a solvent, can dissociate on the Cu surfaces by forming adsorbed CO under H2 atmosphere at 473 K. A small amount of adsorbed CO accelerates Cu redox processes, thus inhibiting catalytic activity with a negative kinetic reaction order. The strong interaction between CO and Cu blocks active sites and disrupts the synergy of Cu+ and Cu0 species, which are vital in DMO hydrogenation. The Ostwald ripening of Cu crystallites is induced by CO, resulting in aggregation of Cu crystallites. The imbalance of active species and crystallite aggregation lead to deactivation of the Cu catalysts during DMO hydrogenation to EG.
中文翻译:

草酸二甲酯加氢制乙二醇过程中SiO 2负载铜催化剂的CO介导失活机理
在基于铜(Cu)的催化剂上,将合成气中的草酸二甲酯(DMO)选择性氢化为乙二醇(EG)是现代合成气化学工业的重要转变。甲醇作为产物或溶剂,可以通过在473 K的H 2气氛下形成吸附的CO来在Cu表面上解离。少量吸附的CO会加速Cu氧化还原过程,从而以负的动力学反应顺序抑制催化活性。CO和Cu之间的强相互作用阻止了活性位点并破坏了Cu +和Cu 0的协同作用在DMO加氢中至关重要的物种。Cu微晶的奥斯特瓦尔德熟化是由CO引起的,导致Cu微晶的聚集。活性物质的不平衡和微晶聚集导致DMO加氢成EG期间Cu催化剂失活。
更新日期:2015-06-09
中文翻译:

草酸二甲酯加氢制乙二醇过程中SiO 2负载铜催化剂的CO介导失活机理
在基于铜(Cu)的催化剂上,将合成气中的草酸二甲酯(DMO)选择性氢化为乙二醇(EG)是现代合成气化学工业的重要转变。甲醇作为产物或溶剂,可以通过在473 K的H 2气氛下形成吸附的CO来在Cu表面上解离。少量吸附的CO会加速Cu氧化还原过程,从而以负的动力学反应顺序抑制催化活性。CO和Cu之间的强相互作用阻止了活性位点并破坏了Cu +和Cu 0的协同作用在DMO加氢中至关重要的物种。Cu微晶的奥斯特瓦尔德熟化是由CO引起的,导致Cu微晶的聚集。活性物质的不平衡和微晶聚集导致DMO加氢成EG期间Cu催化剂失活。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号