Synlett ( IF 1.7 ) Pub Date : 2023-05-11 , DOI: 10.1055/a-2069-3913 Jinjae Park 1 , Tae Lyn Kim 1 , Cheol-Hong Cheon 1
|
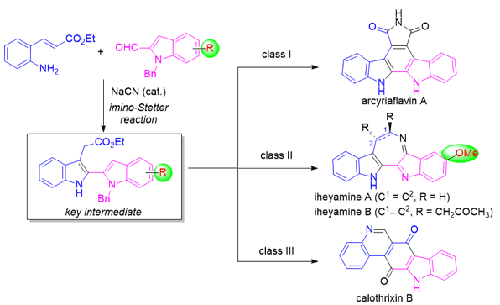
2,2′-Biindolyl natural products have a long history of applications owing to their unique structural features and biological activities. In this Account, we describe the recent progress achieved by our research group in the total syntheses of several 2,2′-biindolyl natural products using the cyanide-catalyzed imino-Stetter reaction as the key reaction to construct the 2,2′-biindolyl scaffold from 2-aminocinnamic acid derivatives and indole-2-carboxaldehydes. The development of a novel protocol to access 2,2′-bisindole-3-acetic acid derivatives via the cyanide-catalyzed imino-Stetter reaction and its application to the total syntheses of class I (arcyriaflavin A), class II (iheyamines A and B), and class III (calothrixin B) 2,2′-biindolyl natural products are discussed.
1. Introduction
2. Synthesis of 2,2′-Biindolyl Compounds via Cyanide-Catalyzed Imino-Stetter Reaction
3. Total Synthesis of Arcyriaflavin A
4. Total Syntheses of Iheyamines A and B
5. Total Synthesis of Calothrixin B
6. Conclusion
中文翻译:

氰化物催化亚氨基-Stetter反应全合成2,2'-联吲哚生物碱
2,2'-联吲哚天然产物因其独特的结构特征和生物活性而具有悠久的应用历史。在这个帐户中,我们描述了我们的研究小组在使用氰化物催化的亚氨基-Stetter 反应作为构建 2,2'-联吲哚基的关键反应的几个 2,2'-联吲哚基天然产物的全合成中取得的最新进展来自 2-氨基肉桂酸衍生物和吲哚-2-甲醛的支架。通过氰化物催化的亚氨基-Stetter 反应获得 2,2'-双吲哚-3-乙酸衍生物的新方案的开发及其在 I 类(arcyriaflavin A)、II 类(iheyamines A 和B) 和 III 类 (calothrixin B) 2,2'-联吲哚天然产物进行了讨论。
一、简介
2. 通过氰化物催化亚氨基-Stetter 反应合成 2,2'-联吲哚化合物
3. Arcyriaflavin A 的全合成
4. Iheyamines A 和 B 的全合成
5. Calothrixin B 的全合成
六,结论







































 京公网安备 11010802027423号
京公网安备 11010802027423号