当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Engineering of the l-Threonine Aldolase from Neptunomonas marine for the Efficient Synthesis of β-Hydroxy-α-amino Acids via C–C Formation
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-12 , DOI: 10.1021/acscatal.3c00672
Yuanzhi He 1 , Siyuan Li 2 , Jun Wang 1 , Xinrui Yang 2 , Jiawei Zhu 1 , Qi Zhang 1 , Li Cui 1 , Zaigao Tan 1 , Wupeng Yan 1 , Yong Zhang 1 , Luyao Tang 1 , Lin-Tai Da 2 , Yan Feng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-12 , DOI: 10.1021/acscatal.3c00672
Yuanzhi He 1 , Siyuan Li 2 , Jun Wang 1 , Xinrui Yang 2 , Jiawei Zhu 1 , Qi Zhang 1 , Li Cui 1 , Zaigao Tan 1 , Wupeng Yan 1 , Yong Zhang 1 , Luyao Tang 1 , Lin-Tai Da 2 , Yan Feng 1
Affiliation
|
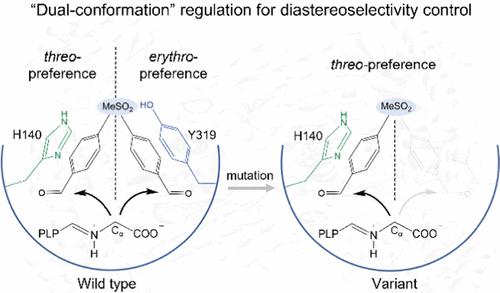
l-Threonine aldolases (LTAs) are attractive biocatalysts for synthesizing β-hydroxy-α-amino acids (HAAs) via C–C bond formation in pharmaceuticals, although their industrial applications suffer from low activity and diastereoselectivity. Herein, we describe the discovery of an LTA from Neptunomonas marine (NmLTA) that displays both ideal enzymatic activity (64.8 U mg–1) and diastereoselectivity (89.5% diastereomeric excess, de) for the desired product l-threo-4-methylsulfonylphenylserine (l-threo-MTPS). Using X-ray crystallography, site-directed mutagenesis, and computational modeling, we propose a “dual-conformation” mechanism for the diastereoselectivity control of NmLTA, whereby the incoming 4-methylsulfonylbenzaldehyde (4-MTB) could potentially bind at the NmLTA active site in two distinct orientations, potentially forming two diastereoisomers (threo- or erythro-form products). Importantly, two key NmLTA residues H140 and Y319 play critical roles in fine-tuning the binding mode of 4-MTB, supported by our site-mutagenesis assays. Uncovering of the catalytic mechanism in NmLTA guides us to further improve the diastereoselectivity of this enzyme. A triple variant of NmLTA (N18S/Q39R/Y319L, SRL) exhibited both improved diastereoselectivity (de value > 99%) and enzymatic activity (95.7 U mg–1) for the synthesis of l-threo-MTPS compared with that of the wild type. The preparative gram-scale synthesis for l-threo-MTPS with the SRL variant produced a space-time yield of up to 9.0 g L–1 h–1, suggesting a potential role as a robust C–C bond synthetic tool for the industrial synthesis of HAAs at a preparative scale. Finally, the SRL variant accepted a wider range of aromatic aldehyde derivatives as substrates and exhibited improved diastereoselectivity toward para-site substituents. This work provides deep structural insights into the molecular mechanism underlying the catalysis in NmLTA and pinpoints the key structural motifs responsible for regulating the diastereoselectivity control, thereby guiding future attempts for protein engineering of various LTAs from different sources.
中文翻译:

发现和工程化来自海王藻的 l-苏氨酸醛缩酶,用于通过 C-C 形成有效合成 β-羟基-α-氨基酸
l -苏氨酸醛缩酶 (LTA) 是一种很有吸引力的生物催化剂,可在药物中通过 C-C 键形成合成 β-羟基-α-氨基酸 (HAA),尽管它们的工业应用存在低活性和非对映选择性的问题。在此,我们描述了从海洋海王星( Nm LTA)中发现的 LTA ,它对所需产物l -苏-4-甲基磺酰苯丝氨酸显示出理想的酶活性 (64.8 U mg –1 ) 和非对映选择性(89.5% 非对映过量,de) ( l -苏-MTPS)。使用 X 射线晶体学、定点诱变和计算模型,我们提出了一种“双构象”机制来控制Nm LTA 的非对映选择性,由此传入的 4-甲基磺酰基苯甲醛 (4-MTB) 可能会在Nm LTA处结合两个不同方向的活性位点,可能形成两种非对映异构体(苏式或赤式产品)。重要的是,两个关键的Nm LTA 残基 H140 和 Y319 在微调 4-MTB 的结合模式中起着关键作用,这得到了我们的位点诱变分析的支持。揭示Nm LTA中的催化机制指导我们进一步提高该酶的非对映选择性。的三重变体与野生型相比,Nm LTA (N18S/Q39R/Y319L, SRL) 在合成l - threo - MTPS 时表现出更高的非对映选择性(de 值 > 99%)和酶活性(95.7 U mg –1 )。具有 SRL 变体的l -苏型-MTPS的制备克级合成产生了高达 9.0 g L –1 h –1的时空产率,这表明其作为工业上强大的 C-C 键合成工具的潜在作用以制备规模合成 HAA。最后,SRL 变体接受更广泛的芳香醛衍生物作为底物,并表现出对对位的改进的非对映选择性-位点取代基。这项工作对Nm LTA 催化的分子机制提供了深入的结构见解,并指出了负责调节非对映选择性控制的关键结构基序,从而指导未来对来自不同来源的各种 LTA 进行蛋白质工程的尝试。
更新日期:2023-05-12
中文翻译:

发现和工程化来自海王藻的 l-苏氨酸醛缩酶,用于通过 C-C 形成有效合成 β-羟基-α-氨基酸
l -苏氨酸醛缩酶 (LTA) 是一种很有吸引力的生物催化剂,可在药物中通过 C-C 键形成合成 β-羟基-α-氨基酸 (HAA),尽管它们的工业应用存在低活性和非对映选择性的问题。在此,我们描述了从海洋海王星( Nm LTA)中发现的 LTA ,它对所需产物l -苏-4-甲基磺酰苯丝氨酸显示出理想的酶活性 (64.8 U mg –1 ) 和非对映选择性(89.5% 非对映过量,de) ( l -苏-MTPS)。使用 X 射线晶体学、定点诱变和计算模型,我们提出了一种“双构象”机制来控制Nm LTA 的非对映选择性,由此传入的 4-甲基磺酰基苯甲醛 (4-MTB) 可能会在Nm LTA处结合两个不同方向的活性位点,可能形成两种非对映异构体(苏式或赤式产品)。重要的是,两个关键的Nm LTA 残基 H140 和 Y319 在微调 4-MTB 的结合模式中起着关键作用,这得到了我们的位点诱变分析的支持。揭示Nm LTA中的催化机制指导我们进一步提高该酶的非对映选择性。的三重变体与野生型相比,Nm LTA (N18S/Q39R/Y319L, SRL) 在合成l - threo - MTPS 时表现出更高的非对映选择性(de 值 > 99%)和酶活性(95.7 U mg –1 )。具有 SRL 变体的l -苏型-MTPS的制备克级合成产生了高达 9.0 g L –1 h –1的时空产率,这表明其作为工业上强大的 C-C 键合成工具的潜在作用以制备规模合成 HAA。最后,SRL 变体接受更广泛的芳香醛衍生物作为底物,并表现出对对位的改进的非对映选择性-位点取代基。这项工作对Nm LTA 催化的分子机制提供了深入的结构见解,并指出了负责调节非对映选择性控制的关键结构基序,从而指导未来对来自不同来源的各种 LTA 进行蛋白质工程的尝试。







































 京公网安备 11010802027423号
京公网安备 11010802027423号