当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photo- and Metal-Mediated Deconstructive Approaches to Cyclic Aliphatic Amine Diversification
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-12 , DOI: 10.1021/jacs.3c01318
David M Soro 1 , Jose B Roque 1 , Jonas W Rackl 1 , Bohyun Park 2, 3 , Stefan Payer 1 , Yuan Shi 4 , J Craig Ruble 4 , Alexey L Kaledin 5 , Mu-Hyun Baik 2, 3 , Djamaladdin G Musaev 5 , Richmond Sarpong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-05-12 , DOI: 10.1021/jacs.3c01318
David M Soro 1 , Jose B Roque 1 , Jonas W Rackl 1 , Bohyun Park 2, 3 , Stefan Payer 1 , Yuan Shi 4 , J Craig Ruble 4 , Alexey L Kaledin 5 , Mu-Hyun Baik 2, 3 , Djamaladdin G Musaev 5 , Richmond Sarpong 1
Affiliation
|
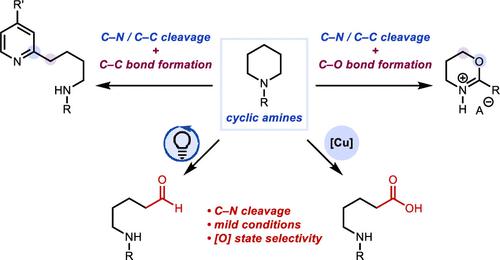
Described herein are studies toward the core modification of cyclic aliphatic amines using either a riboflavin/photo-irradiation approach or Cu(I) and Ag(I) to mediate the process. Structural remodeling of cyclic amines is explored through oxidative C–N and C–C bond cleavage using peroxydisulfate (persulfate) as an oxidant. Ring-opening reactions to access linear aldehydes or carboxylic acids with flavin-derived photocatalysis or Cu salts, respectively, are demonstrated. A complementary ring-opening process mediated by Ag(I) facilitates decarboxylative Csp3–Csp2 coupling in Minisci-type reactions through a key alkyl radical intermediate. Heterocycle interconversion is demonstrated through the transformation of N-acyl cyclic amines to oxazines using Cu(II) oxidation of the alkyl radical. These transformations are investigated by computation to inform the proposed mechanistic pathways. Computational studies indicate that persulfate mediates oxidation of cyclic amines with concomitant reduction of riboflavin. Persulfate is subsequently reduced by formal hydride transfer from the reduced riboflavin catalyst. Oxidation of the cyclic aliphatic amines with a Cu(I) salt is proposed to be initiated by homolysis of the peroxy bond of persulfate followed by α-HAT from the cyclic amine and radical recombination to form an α-sulfate adduct, which is hydrolyzed to the hemiaminal. Investigation of the pathway to form oxazines indicates a kinetic preference for cyclization over more typical elimination pathways to form olefins through Cu(II) oxidation of alkyl radicals.
中文翻译:

光和金属介导的环状脂肪族胺多样化的解构方法
本文描述的是针对使用核黄素/光辐照方法或 Cu(I) 和 Ag(I) 介导该过程的环状脂肪胺核心修饰的研究。使用过氧二硫酸盐(过硫酸盐)作为氧化剂,通过氧化 C-N 和 C-C 键裂解来探索环胺的结构重塑。分别用黄素衍生的光催化或 Cu 盐进行开环反应,以进入线性醛或羧酸。由 Ag(I) 介导的互补开环过程通过关键的烷基自由基中间体促进 Minisci 型反应中的脱羧 Csp3-Csp 2 偶联。通过使用烷基自由基的 Cu(II) 氧化将 N-酰基环胺转化为恶嗪来证明杂环相互转化。通过计算研究这些转换,以告知所提出的机制途径。计算研究表明,过硫酸盐介导环胺的氧化,同时介导核黄素的还原。随后通过从还原核黄素催化剂的正式氢化物转移来还原过硫酸盐。建议通过均解过硫酸盐的过氧键,然后从环胺中产生 α-HAT 和自由基复合形成α-硫酸盐加合物,水解成半氨基,从而引发环状脂肪胺与 Cu(I) 盐的氧化。对形成恶嗪的途径的研究表明,与通过烷基自由基的 Cu(II) 氧化形成烯烃的更典型的消除途径相比,环化的动力学偏好。
更新日期:2023-05-12
中文翻译:

光和金属介导的环状脂肪族胺多样化的解构方法
本文描述的是针对使用核黄素/光辐照方法或 Cu(I) 和 Ag(I) 介导该过程的环状脂肪胺核心修饰的研究。使用过氧二硫酸盐(过硫酸盐)作为氧化剂,通过氧化 C-N 和 C-C 键裂解来探索环胺的结构重塑。分别用黄素衍生的光催化或 Cu 盐进行开环反应,以进入线性醛或羧酸。由 Ag(I) 介导的互补开环过程通过关键的烷基自由基中间体促进 Minisci 型反应中的脱羧 Csp3-Csp 2 偶联。通过使用烷基自由基的 Cu(II) 氧化将 N-酰基环胺转化为恶嗪来证明杂环相互转化。通过计算研究这些转换,以告知所提出的机制途径。计算研究表明,过硫酸盐介导环胺的氧化,同时介导核黄素的还原。随后通过从还原核黄素催化剂的正式氢化物转移来还原过硫酸盐。建议通过均解过硫酸盐的过氧键,然后从环胺中产生 α-HAT 和自由基复合形成α-硫酸盐加合物,水解成半氨基,从而引发环状脂肪胺与 Cu(I) 盐的氧化。对形成恶嗪的途径的研究表明,与通过烷基自由基的 Cu(II) 氧化形成烯烃的更典型的消除途径相比,环化的动力学偏好。

































 京公网安备 11010802027423号
京公网安备 11010802027423号