当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Biological Evaluation and in Silico Studies of 3-Hydroxy-N-(2-(substituted phenyl)-4-oxothiazolidin-3-yl)-2-napthamide Derivatives
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-05-11 , DOI: 10.1002/cbdv.202200976 Shikha Kamboj 1 , Samridhi Thakral 1 , Sunil Kumar 1 , Vikramjeet Singh 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-05-11 , DOI: 10.1002/cbdv.202200976 Shikha Kamboj 1 , Samridhi Thakral 1 , Sunil Kumar 1 , Vikramjeet Singh 1
Affiliation
|
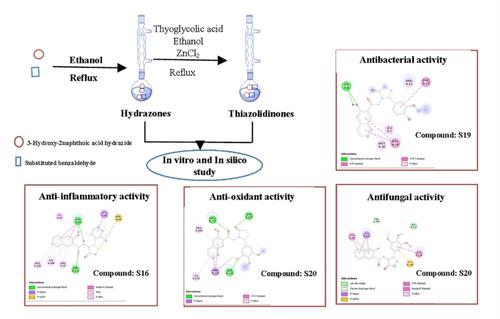
In the present study, a series of 3-hydroxy-N-(2-(substituted phenyl)-4-oxothiazolidin-3-yl)-2-napthamide derivatives were synthesized, characterized and evaluated for theirin vitroactivity, i. e., antimicrobial, antioxidant and anti-inflammatory. The target compounds were synthesized by condensation reaction of 3-hydroxy-2-naphthoic acid hydrazide with substituted benzaldehydes which were subjected to cyclization reaction with thioglycolic acid and ZnCl2 to get target compounds. The synthesized 3-hydroxy-N-(2-(substituted phenyl)-4-oxothiazolidin-3-yl)-2-napthamide derivatives were examined for their antimicrobial activity and 3-hydroxy-N-(4-oxo-2-(3,4,5-trimethoxyphenyl)thiazolidin-3-yl)-2-naphthamide (S20) exhibited the highest antimicrobial potential. The N′-(2,3-dichlorobenzylidene)-3-hydroxy-2-naphthohydrazide (S5) displayed good antifungal potential against Rhizopus oryzae, whereas N′-(2,3-dichlorobenzylidene)-3-hydroxy-2-naphthohydrazide (S20) showed the highest antioxidant potential and N-(2-(2,6-dichlorophenyl)-4-oxothiazolidin-3-yl)-3-hydroxy-2-naphthamide (S16) displayed the highest anti-inflammatory activity. The results of molecular docking studies revealed that existence of hydrogen bonding and hydrophobic interactions with their respective proteins. In silico ADMET studies were carried out by Molinspiration, Pre-ADMET and OSIRIS property explorer to predict the pharmacokinetic behaviour of synthesized 3-hydroxy-N-(2-(substituted phenyl)-4-oxothiazolidin-3-yl)-2-napthamide derivatives.
中文翻译:

3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的合成、生物学评价和计算机模拟研究
在本研究中,合成了一系列3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物,对其体外活性进行了表征和评估。例如,抗菌、抗氧化和抗炎。通过3-羟基-2-萘甲酰肼与取代苯甲醛进行缩合反应合成目标化合物,再与巯基乙酸和ZnCl 2进行环化反应得到目标化合物。检测了合成的 3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的抗菌活性和 3-羟基-N-(4-氧代-2-( 3,4,5-三甲氧基苯基)噻唑烷-3-基)-2-萘酰胺 ( S20)表现出最高的抗菌潜力。N'-(2,3-二氯亚苄基)-3-羟基-2-萘甲酰肼 ( S5 ) 对米根霉表现出良好的抗真菌潜力,而 N'-(2,3-二氯亚苄基)-3-羟基-2-萘甲酰肼 ( S20 ) 显示出最高的抗氧化潜力,N-(2-(2,6-二氯苯基)-4-氧代噻唑烷-3-基)-3-羟基-2-萘酰胺 (S16) 显示出最高的抗炎活性。分子对接研究结果表明其与各自的蛋白质存在氢键和疏水相互作用。计算机模拟通过 Molinspiration、Pre-ADMET 和 OSIRIS property explorer 进行 ADMET 研究,以预测合成的 3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的药代动力学行为。
更新日期:2023-05-11
中文翻译:

3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的合成、生物学评价和计算机模拟研究
在本研究中,合成了一系列3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物,对其体外活性进行了表征和评估。例如,抗菌、抗氧化和抗炎。通过3-羟基-2-萘甲酰肼与取代苯甲醛进行缩合反应合成目标化合物,再与巯基乙酸和ZnCl 2进行环化反应得到目标化合物。检测了合成的 3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的抗菌活性和 3-羟基-N-(4-氧代-2-( 3,4,5-三甲氧基苯基)噻唑烷-3-基)-2-萘酰胺 ( S20)表现出最高的抗菌潜力。N'-(2,3-二氯亚苄基)-3-羟基-2-萘甲酰肼 ( S5 ) 对米根霉表现出良好的抗真菌潜力,而 N'-(2,3-二氯亚苄基)-3-羟基-2-萘甲酰肼 ( S20 ) 显示出最高的抗氧化潜力,N-(2-(2,6-二氯苯基)-4-氧代噻唑烷-3-基)-3-羟基-2-萘酰胺 (S16) 显示出最高的抗炎活性。分子对接研究结果表明其与各自的蛋白质存在氢键和疏水相互作用。计算机模拟通过 Molinspiration、Pre-ADMET 和 OSIRIS property explorer 进行 ADMET 研究,以预测合成的 3-羟基-N-(2-(取代苯基)-4-氧代噻唑烷-3-基)-2-萘酰胺衍生物的药代动力学行为。






































 京公网安备 11010802027423号
京公网安备 11010802027423号