Nano Research ( IF 9.5 ) Pub Date : 2023-05-11 , DOI: 10.1007/s12274-023-5726-7 Hao Wu , Ziyu Song , Wenfang Feng , Zhibin Zhou , Heng Zhang
|
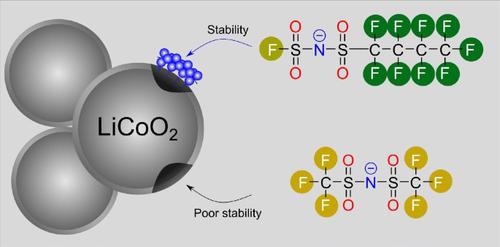
Rechargeable lithium metal batteries (RLMBs) have been regarded as promising successors for contemporary lithium-ion batteries, in view of their high gravimetric and volumetric energy densities. Conventional non-aqueous liquid electrolytes containing organic carbonate solvents possess high chemical reactivities with metallic lithium anode and high flammability, which induces considerable safety threats under extreme conditions (e.g., overcharging and thermal runaway). Herein, we propose the utilization of fluorinated sulfonamide (i.e., N,N-dimethyl fluorosulfonamide (DMFSA)) as solvent, together with lithium (fluorosulfonyl)(n-nonafluorobutanesulfonyl)imide (LiFNFSI) as co-salt and/or electrolyte additive for accessing safer and high-performing RLMBs. Comprehensive physical (e.g., thermal transition, viscosity, and ionic conductivity) and electrochemical (e.g., anodic stability on different electrodes) characterizations have been performed, aiming to reveal the inherent characteristics of the sulfonamide-based electrolytes and the particular role of LiFNFSI on the stabilization of LiCoO2 cathode. It has been demonstrated that the sulfonamide-based electrolytes exhibit superior flame-retardant abilities and decent ionic conductivities (> 1 mS·cm−1 at room temperature). The incorporation of LiFNFSI as co-salt and/or electrolyte additive could significantly suppress the side reactions occurring at the cathode compartment, through the preferential decompositions of the FNFSI− anion. This work is anticipated to give an in-depth understanding on the working mechanism of LiFNFSI in the sulfonamide-based electrolytes, and also spurs the development of high-energy and safer RLMBs.
中文翻译:

锂(氟磺酰基)(正九氟丁基磺酰基)亚胺用于稳定磺酰胺电解质中的阴极-电解质界面
鉴于其高重量和体积能量密度,可充电锂金属电池 (RLMB) 被认为是当代锂离子电池有前途的继任者。含有有机碳酸酯溶剂的传统非水液体电解质与金属锂负极具有高化学反应性和高易燃性,在极端条件下(如过充电和热失控)会引发相当大的安全威胁。在此,我们建议使用氟化磺酰胺(即 N,N-二甲基氟磺酰胺 (DMFSA))作为溶剂,连同锂(氟磺酰基)(n-九氟丁磺酰亚胺 (LiFNFSI) 作为共盐和/或电解质添加剂,用于获得更安全和高性能的 RLMB。已经进行了全面的物理(例如,热转变、粘度和离子电导率)和电化学(例如,不同电极上的阳极稳定性)表征,旨在揭示基于磺胺的电解质的固有特性和 LiFNFSI 在LiCoO 2阴极的稳定性。已经证明,基于磺酰胺的电解质表现出优异的阻燃能力和良好的离子电导率(> 1 mS·cm -1在室温下)。通过优先分解 FNFSI -阴离子,将 LiFNFSI 作为共盐和/或电解质添加剂并入可以显着抑制在阴极室发生的副反应。这项工作有望深入了解 LiFNFSI 在磺胺类电解质中的工作机制,并促进高能和更安全的 RLMB 的发展。






























 京公网安备 11010802027423号
京公网安备 11010802027423号