Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe-Doped Metal Complex (LaCo0.9Fe0.1O3) and g-C3N4 Formed a Nanoheterojunction for the Photocatalytic Decomposition of Water
ACS Omega ( IF 3.7 ) Pub Date : 2023-05-09 , DOI: 10.1021/acsomega.3c01393 Kexin Zhang 1 , Rui Wang 1, 2 , Fubin Jiang 1, 3
ACS Omega ( IF 3.7 ) Pub Date : 2023-05-09 , DOI: 10.1021/acsomega.3c01393 Kexin Zhang 1 , Rui Wang 1, 2 , Fubin Jiang 1, 3
Affiliation
|
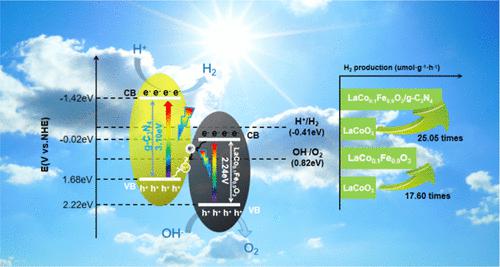
Photocatalytic water decomposition provides an environmentally friendly method of hydrogen production similar to “photosynthesis”, while current research aims to develop affordable yet efficient photocatalysts. Oxygen vacancy is one of the most significant defects in metal oxide semiconductors, including perovskite, which substantially influences the semiconductor material’s efficiency. To enhance the oxygen vacancy in the perovskite, we worked on doping Fe. A perovskite oxide nanostructure of LaCoxFe1–xO3 (x = 0.2, 0.4, 0.6, 0.8, and 0.9) was prepared by the sol–gel method, and a series of LaCoxFe1–xO3 (x = 0.2, 0.4, 0.6, 0.8, and 0.9)/g-C3N4 nanoheterojunction photocatalysts were synthesized using mechanical mixing and solvothermal methods for LaCoxFe1–xO3 (x = 0.2, 0.4, 0.6, 0.8, and 0.9). Fe was successfully doped into the perovskite (LaCoO3), and the formation of an oxygen vacancy was verified by various detection methods. In our photocatalytic water decomposition experiments, we observed that LaCo0.9Fe0.1O3 demonstrated a significant increase in its maximum hydrogen release rate, reaching 5249.21 μmol h–1 g–1, which was remarkably 17.60 times higher than that of LaCoO3-undoped Fe. Similarly, we also explored the photocatalytic activity of the nanoheterojunction complex LaCo0.9Fe0.1O3/g-C3N4, and it exhibited pronounced performance with an average hydrogen production of 7472.67 μmol h–1 g–1, which was 25.05 times that of LaCoO3. We confirmed that the oxygen vacancy plays a crucial role in photocatalysis.
中文翻译:

Fe 掺杂金属配合物 (LaCo0.9Fe0.1O3) 和 g-C3N4 形成用于光催化分解水的纳米异质结
光催化水分解提供了一种类似于“光合作用”的环保制氢方法,而当前的研究旨在开发价格合理但高效的光催化剂。氧空位是金属氧化物半导体(包括钙钛矿)中最重要的缺陷之一,它会极大地影响半导体材料的效率。为了增强钙钛矿中的氧空位,我们致力于掺杂 Fe。采用溶胶-凝胶法制备了LaCo x Fe 1– x O 3 ( x = 0.2, 0.4, 0.6, 0.8, 0.9)钙钛矿氧化物纳米结构,并制备了一系列LaCo x Fe 1– x O 3 ( x= 0.2、0.4、0.6、0.8 和 0.9)/gC 3 N 4纳米异质结光催化剂是使用机械混合和溶剂热法合成的 LaCo x Fe 1– x O 3 ( x = 0.2、0.4、0.6、0.8 和 0.9) . Fe成功地掺杂到钙钛矿(LaCoO 3)中,并通过各种检测方法验证了氧空位的形成。在我们的光催化水分解实验中,我们观察到 LaCo 0.9 Fe 0.1 O 3的最大氢释放速率显着增加,达到 5249.21 μmol h –1 g –1, 是 LaCoO 3 -未掺杂 Fe的 17.60 倍。同样,我们也探索了纳米异质结复合物LaCo 0.9 Fe 0.1 O 3 /gC 3 N 4的光催化活性,它表现出显着的性能,平均产氢量为7472.67 μmol h –1 g –1,是传统复合物的25.05倍。钴酸钴3。我们证实氧空位在光催化中起着至关重要的作用。
更新日期:2023-05-09
中文翻译:

Fe 掺杂金属配合物 (LaCo0.9Fe0.1O3) 和 g-C3N4 形成用于光催化分解水的纳米异质结
光催化水分解提供了一种类似于“光合作用”的环保制氢方法,而当前的研究旨在开发价格合理但高效的光催化剂。氧空位是金属氧化物半导体(包括钙钛矿)中最重要的缺陷之一,它会极大地影响半导体材料的效率。为了增强钙钛矿中的氧空位,我们致力于掺杂 Fe。采用溶胶-凝胶法制备了LaCo x Fe 1– x O 3 ( x = 0.2, 0.4, 0.6, 0.8, 0.9)钙钛矿氧化物纳米结构,并制备了一系列LaCo x Fe 1– x O 3 ( x= 0.2、0.4、0.6、0.8 和 0.9)/gC 3 N 4纳米异质结光催化剂是使用机械混合和溶剂热法合成的 LaCo x Fe 1– x O 3 ( x = 0.2、0.4、0.6、0.8 和 0.9) . Fe成功地掺杂到钙钛矿(LaCoO 3)中,并通过各种检测方法验证了氧空位的形成。在我们的光催化水分解实验中,我们观察到 LaCo 0.9 Fe 0.1 O 3的最大氢释放速率显着增加,达到 5249.21 μmol h –1 g –1, 是 LaCoO 3 -未掺杂 Fe的 17.60 倍。同样,我们也探索了纳米异质结复合物LaCo 0.9 Fe 0.1 O 3 /gC 3 N 4的光催化活性,它表现出显着的性能,平均产氢量为7472.67 μmol h –1 g –1,是传统复合物的25.05倍。钴酸钴3。我们证实氧空位在光催化中起着至关重要的作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号