Nuclear Medicine and Biology ( IF 3.6 ) Pub Date : 2023-05-08 , DOI: 10.1016/j.nucmedbio.2023.108350 Yun Xi 1 , Hong Chen 1 , Yue Xi 1 , Wangxi Hai 1 , Qian Qu 1 , Min Zhang 1 , Biao Li 1
|
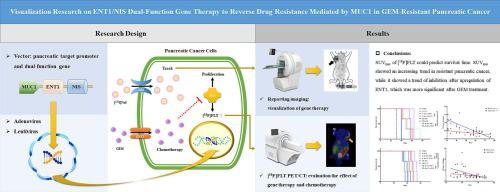
Purpose
To use bifunctional target genes to increase the intracellular transport of gemcitabine (GEM) to reverse chemotherapy resistance and to simultaneously use reporter gene imaging to localize therapeutic genes. The therapeutic effect was evaluated by [18F]FLT PET/CT to visualize the effect of gene therapy.
Methods
A viral gene vector containing the pancreatic cancer-targeting promoter MUC1 for specific transcription of equilibrative nucleoside transporter 1 (ENT1) and NIS (nuclide transport channel) was employed. [125I]NaI uptake tests and [131I]NaI SPECT imaging were performed to verify the function of NIS and the target function of MUC1. The correlation between [18F]FLT uptake and GEM resistance were assessed, and the influence ENT1 and thymidine kinase 1 (TK1) expression on [18F]FLT micro-PET/CT was measured, which provides a theoretical basis for the use of [18F]FLT micro-PET/CT to evaluate the efficacy of gene therapy.
Results
First, functions of gene therapy were confirmed: ENT1 reversed the drug resistance of GEM-resistant pancreatic cancer cells by increasing GEM intracellular transport; MUC1 drove NIS target gene expression in pancreatic cancer; and therapeutic genes could be localized using [131I]NaI SPECT reporter gene imaging. Second, the [18F]FLT uptake ratio was affected by drug resistance and GEM treatment. The mechanism underlying this effect was related to ENT1 and TK1. Increased expression of ENT1 inhibited the expression of TK1 after GEM chemotherapy to reduce the uptake of [18F]FLT. Finally, micro-PET/CT indicated that the SUVmax of [18F]FLT could predict survival time. SUVmax exhibited an increasing trend in resistant pancreatic cancer but a trend of inhibition after upregulation of ENT1, which was more significant after GEM treatment.
Conclusions
Bifunctional targeted genes can localize therapeutic genes through reporter gene imaging, reverse the drug resistance of GEM-resistant pancreatic cancer and be visually evaluated through [18F]FLT micro-PET/CT.
中文翻译:

ENT1/NIS双功能基因治疗逆转MUC1介导的GEM耐药胰腺癌耐药的可视化研究
目的
使用双功能靶基因增加吉西他滨(GEM)的细胞内转运以逆转化疗耐药性,并同时使用报告基因成像来定位治疗基因。通过[ 18 F]FLT PET/CT评估治疗效果,以可视化基因治疗的效果。
方法
采用含有胰腺癌靶向启动子 MUC1 的病毒基因载体,用于平衡核苷转运蛋白 1 (ENT1) 和 NIS(核素转运通道)的特异性转录。进行[ 125 I]NaI摄取测试和[ 131 I]NaI SPECT成像来验证NIS的功能和MUC1的靶功能。评估[ 18 F]FLT摄取与GEM耐药之间的相关性,并测定ENT1和胸苷激酶1(TK1)表达对[ 18 F]FLT micro-PET/CT的影响,为使用[ 18 F]FLT提供理论依据。 [ 18 F]FLT micro-PET/CT评估基因治疗的疗效。
结果
首先,基因治疗的功能得到证实:ENT1通过增加GEM细胞内转运,逆转GEM耐药胰腺癌细胞的耐药性; MUC1驱动胰腺癌中NIS靶基因表达;可以使用[ 131 I]NaI SPECT 报告基因成像来定位治疗基因。其次,[ 18 F]FLT摄取率受耐药性和GEM治疗的影响。这种效应的机制与 ENT1 和 TK1 有关。 GEM化疗后ENT1表达增加抑制TK1表达,从而减少[ 18 F]FLT的摄取。最后,微型PET/CT表明[ 18 F]FLT的SUV max可以预测生存时间。 SUV max在耐药胰腺癌中呈现增加趋势,但在 ENT1 上调后呈现抑制趋势,且在 GEM 治疗后更为显着。
结论
双功能靶向基因可以通过报告基因成像定位治疗基因,逆转GEM耐药胰腺癌的耐药性,并通过[ 18 F]FLT micro-PET/CT进行直观评估。































 京公网安备 11010802027423号
京公网安备 11010802027423号