Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-05-08 , DOI: 10.1016/j.cclet.2023.108540 Yini Mao , Yong Jiang , Hao Liu , Yimin Jiang , Ming Li , Wei Su , Rongxing He
|
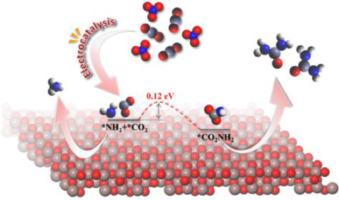
Urea plays a vital role in the sustainable development of mankind as it is one of the most important nitrogen fertilizers. Conventional synthesis of urea is accompanied by a high level of energy consumption while electrocatalytic methods suffer from low yields and poor selectivity. Our work achieves efficient synthesis of urea by designing the graphene-In2O3 electrocatalysts for the co-activated reduction of nitrate and carbon dioxide, where the formation rate of urea, Faraday efficiency (FE) and carbon selectivity at -0.35 V vs. RHE can reach 357.47 µg mg−1 h−1, 10.46% and ∼100%, respectively. Herein, the key intermediates in the C N coupling reaction are demonstrated to be *NH2 and *CO2, which is of novelty compared to previous reports. This work may provide inspiration for subsequent studies on the reaction mechanism of the electrochemical synthesis of urea, as well as theoretical guidance for the sustainable synthesis of some other important chemical substances.
N coupling reaction are demonstrated to be *NH2 and *CO2, which is of novelty compared to previous reports. This work may provide inspiration for subsequent studies on the reaction mechanism of the electrochemical synthesis of urea, as well as theoretical guidance for the sustainable synthesis of some other important chemical substances.
中文翻译:

在石墨烯负载的 In2O3 上通过 NO3− 和 CO2 共还原进行尿素的常温电催化合成
尿素是最重要的氮肥之一,对人类可持续发展发挥着至关重要的作用。传统的尿素合成方法能耗高,而电催化方法则收率低、选择性差。我们的工作通过设计用于共激活硝酸盐和二氧化碳还原的石墨烯-In 2 O 3电催化剂实现了尿素的高效合成,其中尿素的形成速率、法拉第效率(FE)和碳选择性在-0.35 V vs. RHE可分别达到357.47 µg mg −1 h −1、10.46%和∼100%。在此,C N偶联反应中的关键中间体被证明是*NH 2和*CO 2 ,这与之前的报道相比具有新颖性。该工作可能为后续尿素电化学合成反应机理的研究提供启发,也为其他一些重要化学物质的可持续合成提供理论指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号