European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-05-08 , DOI: 10.1016/j.ejmech.2023.115470 Hui Wu 1 , Long-Shan Wang 1 , Pei Li 2 , Jia Yu 2 , Sha Cheng 2 , Gang Yu 2 , Mashaal Ahmad 2 , Xue-Ling Meng 2 , Heng Luo 2 , Bi-Xue Xu 2
|
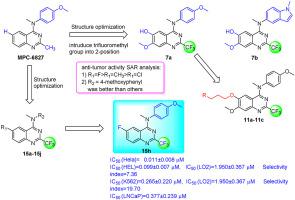
A series of new N-aryl-2-trifluoromethylquinazoline-4-amine analogs were designed and synthesized based on structure optimization of quinazoline by introducing a trifluoromethyl group into 2-position. The structures of the twenty-four newly synthesized compounds were confirmed by 1H NMR, 13C NMR and ESI-MS. The in vitro anti-cancer activity against chronic myeloid leukemia cells (K562), erythroleukemia cells (HEL), human prostate cancer cells (LNCaP), and cervical cancer cells (HeLa) of the target compounds was evaluated. Among them, compounds 15d, 15f, 15h, and 15i showed the significantly (P < 0.01) stronger growth inhibitory activity on K562 than those of the positive controls of paclitaxel and colchicine, while compounds 15a, 15d, 15e, and 15h displayed significantly stronger growth inhibitory activity on HEL than those of the positive controls. However, all the target compounds exhibited weaker growth inhibition activity against K562 and HeLa than those of the positive controls. The selectivity ratio of compounds 15h, 15d, and 15i were significantly higher than those of other active compounds, indicating that these three compounds had the lower hepatotoxicity. Several compounds displayed strong inhibition against leukemia cells. They inhibited tubulin polymerization, disrupted cellular microtubule networks by targeting the colchicine site, and promoted cell cycle arrest of leukemia cells at G2/M phase and cell apoptosis, as well as inhibiting angiogenesis. In summary, our research provided that novel synthesized N-aryl-2-trifluoromethyl-quinazoline-4-amine active derivatives as the inhibitors of tubulin polymerization in leukemia cells, which might be a valuable lead compounds for anti-leukemia agents.
中文翻译:

发现新型 N-aryl-2-trifluoromethyl-quinazoline-4-amine 衍生物作为白血病细胞微管蛋白聚合的抑制剂
在喹唑啉结构优化的基础上,通过在2-位引入三氟甲基,设计合成了一系列新的N-芳基-2-三氟甲基喹唑啉-4-胺类似物。新合成的24个化合物的结构均通过1 H NMR、13 C NMR和ESI-MS确证。评估了目标化合物对慢性粒细胞白血病细胞(K562)、红白血病细胞(HEL)、人前列腺癌细胞(LNCaP)和宫颈癌细胞(HeLa)的体外抗癌活性。其中化合物15d、15f、15h和15i表现出显着的( P < 0.01)对K562的生长抑制活性强于紫杉醇和秋水仙碱阳性对照,而化合物15a、15d、15e和15h对HEL的生长抑制活性明显强于阳性对照。然而,所有目标化合物对 K562 和 HeLa 的生长抑制活性均低于阳性对照。化合物15h , 15d , 15i的选择性比显着高于其他活性化合物,表明这三种化合物具有较低的肝毒性。几种化合物显示出对白血病细胞的强烈抑制作用。它们抑制微管蛋白聚合,通过靶向秋水仙碱位点破坏细胞微管网络,促进白血病细胞周期停滞在 G2/M 期和细胞凋亡,以及抑制血管生成。总之,我们的研究提供了新型合成的N -aryl-2-trifluoromethyl-quinazoline-4-amine 活性衍生物作为白血病细胞微管蛋白聚合的抑制剂,这可能是一种有价值的抗白血病药物先导化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号