当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Evaluation of First-in-Class Dual Acting Histone Deacetylases (HDACs) and Phosphodiesterase 5 (PDE5) Inhibitors for the Treatment of Alzheimer’s Disease
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-09-27 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00908 Obdulia Rabal , Juan A. Sánchez-Arias , Mar Cuadrado-Tejedor 1 , Irene de Miguel , Marta Pérez-González , Carolina García-Barroso , Ana Ugarte , Ander Estella-Hermoso de Mendoza , Elena Sáez , Maria Espelosin , Susana Ursua , Tan Haizhong 2 , Wu Wei 2 , Xu Musheng 2 , Ana Garcia-Osta , Julen Oyarzabal
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2016-09-27 00:00:00 , DOI: 10.1021/acs.jmedchem.6b00908 Obdulia Rabal , Juan A. Sánchez-Arias , Mar Cuadrado-Tejedor 1 , Irene de Miguel , Marta Pérez-González , Carolina García-Barroso , Ana Ugarte , Ander Estella-Hermoso de Mendoza , Elena Sáez , Maria Espelosin , Susana Ursua , Tan Haizhong 2 , Wu Wei 2 , Xu Musheng 2 , Ana Garcia-Osta , Julen Oyarzabal
Affiliation
|
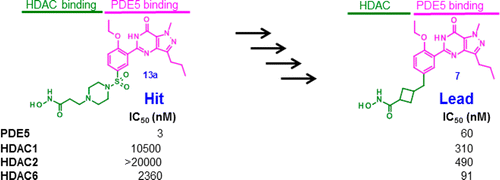
Simultaneous inhibition of phosphodiesterase 5 (PDE5) and histone deacetylases (HDAC) has recently been validated as a potentially novel therapeutic approach for Alzheimer’s disease (AD). To further extend this concept, we designed and synthesized the first chemical series of dual acting PDE5 and HDAC inhibitors, and we validated this systems therapeutics approach. Following the implementation of structure- and knowledge-based approaches, initial hits were designed and were shown to validate our hypothesis of dual in vitro inhibition. Then, an optimization strategy was pursued to obtain a proper tool compound for in vivo testing in AD models. Initial hits were translated into molecules with adequate cellular functional responses (histone acetylation and cAMP/cGMP response element-binding (CREB) phosphorylation in the nanomolar range), an acceptable therapeutic window (>1 log unit), and the ability to cross the blood–brain barrier, leading to the identification of 7 as a candidate for in vivo proof-of-concept testing (Cuadrado-Tejedor, M.; Garcia-Barroso, C.; Sánchez-Arias, J. A.; Rabal, O.; Mederos, S.; Ugarte, A.; Franco, R.; Segura, V.; Perea, G.; Oyarzabal, J.; Garcia-Osta, A. Neuropsychopharmacology 2016, in press, doi: 10.1038/npp.2016.163).
中文翻译:

用于阿尔茨海默氏病治疗的一流双作用组蛋白脱乙酰基酶(HDAC)和磷酸二酯酶5(PDE5)抑制剂的设计,合成和生物学评估
磷酸二酯酶5(PDE5)和组蛋白脱乙酰基酶(HDAC)的同时抑制最近已被证实是阿尔茨海默氏病(AD)潜在的新型治疗方法。为了进一步扩展这个概念,我们设计并合成了第一个化学作用的双作用PDE5和HDAC抑制剂系列,并验证了该系统的治疗方法。在实施基于结构和知识的方法之后,设计了初始命中并显示了其来验证我们的双重体外抑制假说。然后,寻求优化策略以获得适合体内的工具化合物在AD模型中进行测试。最初的命中被翻译成具有适当细胞功能反应(组蛋白乙酰化和cAMP / cGMP反应元件结合(CREB)磷酸化在纳摩尔范围内),可接受的治疗窗(> 1 log单位)和穿越血液的能力的分子–脑屏障,从而将7鉴定为体内概念验证测试的候选者(Cuadrado-Tejedor,M。Garcia-Barroso,C .;JA,Sánchez-Arias;俄克拉荷马州拉巴尔;梅德罗斯(Mederos),S .;乌加特(A.)佛朗哥(Franco,R.)塞古拉(Segura)佩里亚(Perea)J.Oyarzabal;加西亚·奥斯塔(A. Neuropsychopharmacology 2016,印刷中,doi:10.1038 / npp.2016.163)。
更新日期:2016-09-27
中文翻译:

用于阿尔茨海默氏病治疗的一流双作用组蛋白脱乙酰基酶(HDAC)和磷酸二酯酶5(PDE5)抑制剂的设计,合成和生物学评估
磷酸二酯酶5(PDE5)和组蛋白脱乙酰基酶(HDAC)的同时抑制最近已被证实是阿尔茨海默氏病(AD)潜在的新型治疗方法。为了进一步扩展这个概念,我们设计并合成了第一个化学作用的双作用PDE5和HDAC抑制剂系列,并验证了该系统的治疗方法。在实施基于结构和知识的方法之后,设计了初始命中并显示了其来验证我们的双重体外抑制假说。然后,寻求优化策略以获得适合体内的工具化合物在AD模型中进行测试。最初的命中被翻译成具有适当细胞功能反应(组蛋白乙酰化和cAMP / cGMP反应元件结合(CREB)磷酸化在纳摩尔范围内),可接受的治疗窗(> 1 log单位)和穿越血液的能力的分子–脑屏障,从而将7鉴定为体内概念验证测试的候选者(


















































 京公网安备 11010802027423号
京公网安备 11010802027423号