Journal of Materials Science & Technology ( IF 11.2 ) Pub Date : 2023-05-07 , DOI: 10.1016/j.jmst.2023.03.040 Xiao Li , Yigang Yan , Torben R. Jensen , Yaroslav Filinchuk , Iurii Dovgaliuk , Dmitry Chernyshov , Liqing He , Yongtao Li , Hai-Wen Li
|
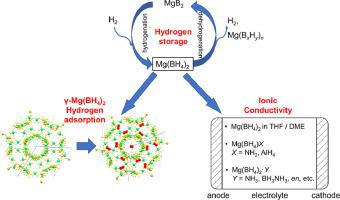
Mg(BH4)2 with several polymorphs, known as a high capacity (14.9 wt.%) hydrogen storage material, has become more intriguing due to the recently found new functions of gas physisorption and ionic conductivity. Here we review the state-of-the-art on the energy related functions of Mg(BH4)2. Mg(BH4)2 tends to form the stable intermediate [B12H12]2− when the dehydrogenation temperature is above 400 °C, the strong B-B bonding of which makes the rehydrogenation condition very harsh. In contrast, lower borane intermediate [B3H8]2− facilitates the rehydrogenation even at a mild condition of 100 °C, suggesting the possibility of reversible hydrogen storage in Mg(BH4)2. The porous polymorph γ-Mg(BH4)2 shows attractive gas adsorption properties in view of its unique hydridic surface and pore shape, and potentially can be applied in hydrogen adsorption and Kr/Xe selectivity. A new diffraction-based adsorption methodology was developed to characterize adsorption thermodynamics and kinetics of γ-Mg(BH4)2, providing a novel idea for the characterization of crystalline porous materials. Moreover, the potential of Mg(BH4)2 as an electrolyte is discussed in the last part. Mg(BH4)2·THF/DME acts as a liquid electrolyte in Mg-batteries, while anion substituted or neutral molecule derivatives of Mg(BH4)2 can act as solid-state electrolyte.
中文翻译:

用于能源应用的硼氢化镁 Mg(BH4)2:综述
Mg(BH 4 ) 2具有多种晶型,被称为高容量(14.9 wt.%)储氢材料,由于最近发现的气体物理吸附和离子电导率的新功能而变得更加有趣。在这里,我们回顾了 Mg(BH 4 ) 2的能量相关功能的最新技术。当脱氢温度高于400℃时,Mg(BH 4 ) 2倾向于形成稳定的中间体[B 12 H 12 ] 2− ,其强BB键使再氢化条件非常苛刻。相反,较低的硼烷中间体 [B 3 H 8 ] 2−即使在 100 °C 的温和条件下也能促进再氢化,表明 Mg(BH 4 ) 2中可逆储氢的可能性。鉴于其独特的氢化表面和孔形状,多孔多晶型物 γ-Mg(BH 4 ) 2显示出诱人的气体吸附性能,并可能应用于氢吸附和 Kr/Xe 选择性。开发了一种新的基于衍射的吸附方法来表征γ-Mg(BH 4 ) 2的吸附热力学和动力学,为结晶多孔材料的表征提供了新思路。此外,Mg(BH 4 ) 2的潜力作为电解质在最后一部分讨论。Mg(BH 4 ) 2 ·THF/DME在镁电池中充当液态电解质,而Mg(BH 4 ) 2的阴离子取代或中性分子衍生物可充当固态电解质。








































 京公网安备 11010802027423号
京公网安备 11010802027423号