Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cannabicitran: Its unexpected racemic nature and potential origins
Chirality ( IF 2.8 ) Pub Date : 2023-05-04 , DOI: 10.1002/chir.23571 Jared S Wood 1 , William H Gordon 1 , Jeremy B Morgan 1 , R Thomas Williamson 1
Chirality ( IF 2.8 ) Pub Date : 2023-05-04 , DOI: 10.1002/chir.23571 Jared S Wood 1 , William H Gordon 1 , Jeremy B Morgan 1 , R Thomas Williamson 1
Affiliation
|
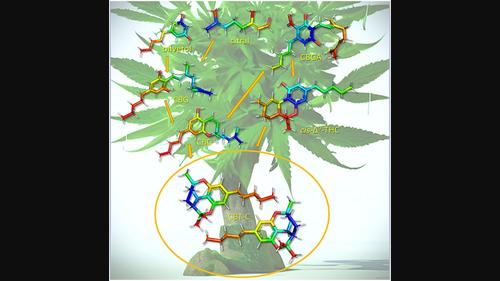
Cannabicitran is a cannabinoid found in levels up to ~10% in commercial “purified” cannabidiol (CBD) extracts. The structure of this natural product was first reported more than 50 years ago. However, few studies have investigated cannabicitran or its origin despite the rapidly increasing interest in the use of cannabinoids for the treatment of a wide range of physiological conditions. Following on a recent detailed NMR and computational characterization of cannabicitran, our group initiated ECD and TDDFT studies aimed at unequivocally determining the absolute configuration of cannabicitran present in Cannabis sativa extracts. To our surprise, we discovered the natural product was racemic, raising questions around its presumed enzymatic origin. Herein, we report the isolation and absolute configuration of (−)-cannabicitran and (+)-cannabicitran. Several possible scenarios for production of the racemate in the plant and/or during extract processing are discussed.
中文翻译:

大麻素:其意想不到的外消旋性质和潜在起源
Cannabicitran 是一种大麻素,在商业“纯化”大麻二酚 (CBD) 提取物中含量高达约 10%。这种天然产物的结构在 50 多年前首次被报道。然而,尽管人们对使用大麻素治疗各种生理状况的兴趣迅速增加,但很少有研究调查大麻西群或其起源。在最近对大麻素进行了详细的 NMR 和计算表征之后,我们的小组启动了 ECD 和 TDDFT 研究,旨在明确确定大麻提取物中存在的大麻素的绝对构型。令我们惊讶的是,我们发现天然产物是外消旋的,这引发了人们对其推测的酶起源的质疑。在此,我们报告了 (−)-cannabicitran 和 (+)-cannabicitran 的分离和绝对构型。讨论了在植物中和/或在提取物加工过程中生产外消旋体的几种可能的情况。
更新日期:2023-05-04
中文翻译:

大麻素:其意想不到的外消旋性质和潜在起源
Cannabicitran 是一种大麻素,在商业“纯化”大麻二酚 (CBD) 提取物中含量高达约 10%。这种天然产物的结构在 50 多年前首次被报道。然而,尽管人们对使用大麻素治疗各种生理状况的兴趣迅速增加,但很少有研究调查大麻西群或其起源。在最近对大麻素进行了详细的 NMR 和计算表征之后,我们的小组启动了 ECD 和 TDDFT 研究,旨在明确确定大麻提取物中存在的大麻素的绝对构型。令我们惊讶的是,我们发现天然产物是外消旋的,这引发了人们对其推测的酶起源的质疑。在此,我们报告了 (−)-cannabicitran 和 (+)-cannabicitran 的分离和绝对构型。讨论了在植物中和/或在提取物加工过程中生产外消旋体的几种可能的情况。































 京公网安备 11010802027423号
京公网安备 11010802027423号