Synthesis ( IF 2.2 ) Pub Date : 2023-05-04 , DOI: 10.1055/s-0042-1751446 Zachary Schwartz , Chelsea Valiton , Myles Lovasz , Andrew G. Roberts
|
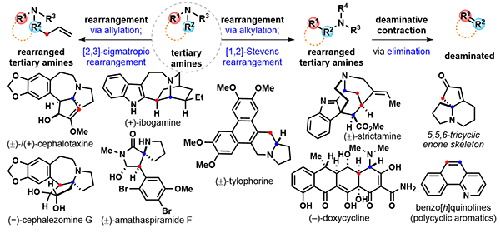
Ammonium ylide based [2,3]-sigmatropic and [1,2]-Stevens rearrangements enable the transformation of tertiary amines into rearranged and functionalized intermediates en route to many polycyclic natural product targets. Herein, we summarize recent applications of these rearrangement reactions in formal and total synthesis endeavors while highlighting innovative improvements to these transforms.
1 Introduction
2 Ammonium Ylide Based [2,3]-Sigmatropic Rearrangements in Natural Product Synthesis
2.1 (–)-Cephalotaxine
2.2 (±)-Amathaspiramide F
2.3 (–)-Cephalezomine G and Its C3 Epimer
2.4 (±)-Strictamine
2.5 (–)-Doxycycline
3 [1,2]-Stevens Rearrangements Toward Natural Products
3.1 Ring-Expanding [1,2]-Stevens Rearrangements en route to (±)-Tylophorine, (±)-7-Methoxycryptopleurine, and (±)-Xylopinine
3.2 Enantioselective Synthesis of Iboga Alkaloids and (+)-Vinblastine
4 Selected Methodology
4.1 Ammonium Ylide Based [2,3]-Sigmatropic Rearrangements To Form Natural Product Cores
4.2 Cascade Reactions Involving [1,2]-Stevens Rearrangement/ Hofmann-Type Elimination Events
5 Conclusions
中文翻译:

基于铵叶立德的 [2,3]-Sigmatropic 和 [1,2]-Stevens 重排将胺转化为天然产物的最新应用
基于铵叶立德的 [2,3]-sigmatropic 和 [1,2]-Stevens 重排能够将叔胺转化为重排和功能化的中间体,从而达到许多多环天然产物目标。在此,我们总结了这些重排反应在形式和全合成方面的最新应用,同时强调了对这些转化的创新改进。
1 简介
2 基于铵叶立德的[2,3]-Sigmatropic 重排在天然产物合成中的应用
2.1 (–)-头孢紫杉醇
2.2 (±)-Amathaspiramide F
2.3 (–)-头孢佐明G及其C3差向异构体
2.4 (±)-黑胡桃胺
2.5 (–)-多西环素
3 [1,2]-Stevens 对天然产物的重排
3.1 扩环 [1,2]-Stevens 重排生成(±)-Tylophorine、(±)-7-Methoxycryptopleurine 和 (±)-Xylopinine
3.2 伊波加生物碱与(+)-长春碱的对映选择性合成
4 选定的方法论
4.1 基于铵叶立德的 [2,3]-Sigmatropic 重排形成天然产物核心
4.2 涉及[1,2]-史蒂文斯重排/霍夫曼型消除事件的级联反应
5。结论






























 京公网安备 11010802027423号
京公网安备 11010802027423号