当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competition between the Ni and Fe Redox in the O3-NaNi1/3Fe1/3Mn1/3O2 Cathode Material for Na-Ion Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-05-04 , DOI: 10.1021/acs.chemmater.3c00338 Vitalii A. Shevchenko 1, 2 , Iana S. Glazkova 1, 3 , Daniil A. Novichkov 1 , Irina Skvortsova 2, 4 , Alexey V. Sobolev 1, 3 , Artem M. Abakumov 2 , Igor A. Presniakov 1 , Oleg A. Drozhzhin 1 , Evgeny V. Antipov 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-05-04 , DOI: 10.1021/acs.chemmater.3c00338 Vitalii A. Shevchenko 1, 2 , Iana S. Glazkova 1, 3 , Daniil A. Novichkov 1 , Irina Skvortsova 2, 4 , Alexey V. Sobolev 1, 3 , Artem M. Abakumov 2 , Igor A. Presniakov 1 , Oleg A. Drozhzhin 1 , Evgeny V. Antipov 1, 2
Affiliation
|
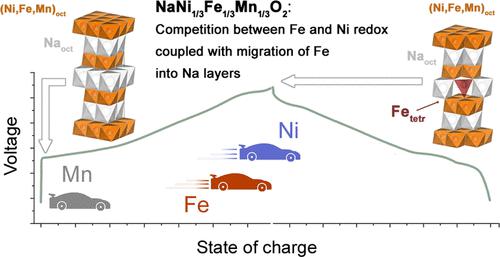
Sodium-ion batteries are attracting great attention due to their low cost and abundance of sodium. The O3-type NaNi1/3Fe1/3Mn1/3O2 layered oxide material is a promising candidate for positive electrodes (cathodes) in Na-ion batteries. However, its stable electrochemical performance is restricted by the upper voltage limit of 4.0 V (vs Na/Na+), which allows for reversibly removing 0.5–0.55 Na+ per formula unit, corresponding to the capacity of 120–130 mAh·g–1. Further reduction of sodium content inevitably accelerates capacity degradation, and this issue calls for a detailed study of the redox reactions that accompany the electrochemical (de)intercalation of a large amount of sodium. Here, we present operando and ex situ studies using powder X-ray diffraction and X-ray absorption spectroscopy combined with 57Fe Mössbauer spectroscopy. Our approach reveals the sequence of the redox transitions that occur during the charge and discharge of O3-NaNi1/3Fe1/3Mn1/3O2. Our data show that in addition to nickel and iron cations oxidizing to M+4, a part of iron transforms into the “3 + δ” state owing to the fast electron exchange Fe3+ + Fe4+ ↔ Fe4+ + Fe3+. This process freezes upon cooling the material to 35 K, producing Fe4+ cations, some of which occupy tetrahedral positions.
中文翻译:

O3-NaNi1/3Fe1/3Mn1/3O2钠离子电池正极材料中Ni和Fe氧化还原的竞争
钠离子电池因其低成本和丰富的钠元素而备受关注。O3型NaNi 1/3 Fe 1/3 Mn 1/3 O 2层状氧化物材料是一种很有前途的钠离子电池正极(正极)材料。然而,其稳定的电化学性能受到电压上限 4.0 V(vs Na/Na +)的限制,这允许每个配方单元可逆地去除 0.5–0.55 Na +,对应于 120–130 mAh·g 的容量– 1个. 进一步降低钠含量不可避免地会加速容量退化,这个问题需要详细研究伴随大量钠的电化学(脱)嵌入的氧化还原反应。在这里,我们使用粉末 X 射线衍射和 X 射线吸收光谱结合57 Fe Mössbauer 光谱进行原位和非原位研究。我们的方法揭示了 O3-NaNi 1/3 Fe 1/3 Mn 1/3 O 2充电和放电期间发生的氧化还原转变的顺序。我们的数据表明,除了镍和铁阳离子氧化成 M +4外,部分铁由于快速电子交换 Fe 3+而转变为“3 + δ”状态+ Fe 4+ ↔ Fe 4+ + Fe 3+。该过程在将材料冷却至 35 K 时冻结,产生 Fe 4+阳离子,其中一些占据四面体位置。
更新日期:2023-05-04
中文翻译:

O3-NaNi1/3Fe1/3Mn1/3O2钠离子电池正极材料中Ni和Fe氧化还原的竞争
钠离子电池因其低成本和丰富的钠元素而备受关注。O3型NaNi 1/3 Fe 1/3 Mn 1/3 O 2层状氧化物材料是一种很有前途的钠离子电池正极(正极)材料。然而,其稳定的电化学性能受到电压上限 4.0 V(vs Na/Na +)的限制,这允许每个配方单元可逆地去除 0.5–0.55 Na +,对应于 120–130 mAh·g 的容量– 1个. 进一步降低钠含量不可避免地会加速容量退化,这个问题需要详细研究伴随大量钠的电化学(脱)嵌入的氧化还原反应。在这里,我们使用粉末 X 射线衍射和 X 射线吸收光谱结合57 Fe Mössbauer 光谱进行原位和非原位研究。我们的方法揭示了 O3-NaNi 1/3 Fe 1/3 Mn 1/3 O 2充电和放电期间发生的氧化还原转变的顺序。我们的数据表明,除了镍和铁阳离子氧化成 M +4外,部分铁由于快速电子交换 Fe 3+而转变为“3 + δ”状态+ Fe 4+ ↔ Fe 4+ + Fe 3+。该过程在将材料冷却至 35 K 时冻结,产生 Fe 4+阳离子,其中一些占据四面体位置。































 京公网安备 11010802027423号
京公网安备 11010802027423号