Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-05-04 , DOI: 10.1016/j.molliq.2023.122000 Qiujie Wang , Xiaoxiao Li , Liying Song , Jinfeng Zhao , Zhe Tang
|
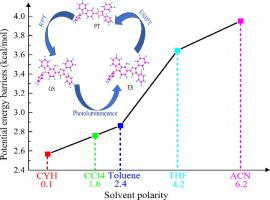
It is necessary to study the excited-state intramolecular proton transfer of complex molecular systems in solvents, which play important roles in photochemistry, photobiology, and optoelectronic materials. In this work, we have worked on the effect of solvent polarity on the ESIPT behaviour of 3-(4,5-Diphenyl-1H-imidazol-2-yl)-9-phenyl-9,9a-dihydro-4aH-carbazol-4-ol (CHPI). Specifically, an in-depth study of hydrogen bonding behavior and charge complexation in different polar solvents has confirmed the excited state hydrogen bonding enhancement mechanism, which is the primary prerequisite to facilitate the ESIPT process. It is noteworthy that the intramolecular hydrogen bonding of CHPI compound is enhanced more significantly in nonpolar solvents. To further elaborate the ESIPT process, we analyzed the potential energy curves of the S0 and S1 states, and the comparison shows that the potential barrier decreases as the solvent polarity decreases. Therefore, the nonpolar solvent environment is more favorable for CHPI molecule to undergo the ESIPT process.
中文翻译:

3-(4,5-二苯基-1H-咪唑-2-基)-9-苯基-9H-咔唑-4-醇化合物在不同溶剂中ESIPT机理及负溶剂化显色效应的理论研究
有必要研究复杂分子系统在溶剂中的激发态分子内质子转移,这在光化学、光生物学和光电材料中起着重要作用。在这项工作中,我们研究了溶剂极性对 3-(4,5-Diphenyl-1H-imidazol-2-yl)-9-phenyl-9,9a-dihydro-4aH-carbazol- ESIPT 行为的影响。 4-醇 (CHPI)。具体而言,对不同极性溶剂中氢键行为和电荷络合的深入研究证实了激发态氢键增强机制,这是促进 ESIPT 过程的首要前提。值得注意的是,CHPI 化合物的分子内氢键在非极性溶剂中更显着增强。为了进一步阐述 ESIPT 过程,我们分析了S0和S1态的势能曲线,比较表明势垒随着溶剂极性的降低而降低。因此,非极性溶剂环境更有利于CHPI分子进行ESIPT过程。














































 京公网安备 11010802027423号
京公网安备 11010802027423号